 Investor Relations Contact: Nasdaq: JAN
Investor Relations Contact: Nasdaq: JANExhibit 99.1
 Investor Relations Contact: Nasdaq: JAN
Investor Relations Contact: Nasdaq: JAN
(800) 400-2247 www. janone.com
IR@janone.com
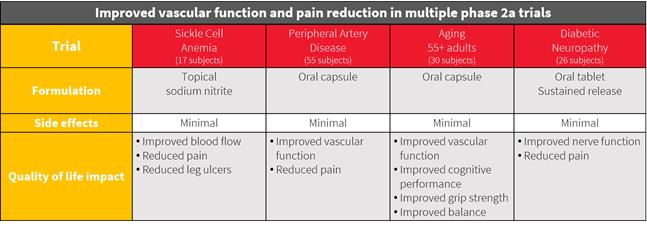
JanOne (Nasdaq: JAN) is a biopharma company uniquely focused on developing medications to treat diseases that cause severe pain, with its initial focus on vascular conditions such as Peripheral Artery Disease (PAD). By alleviating disease associated pain at the source, this may also reduce the need for opioid prescriptions often used to treat severe pain, thus can potentially lead to an impact on prescription opioid abuse. To that end, the company is also exploring solutions for non-addictive acute pain medications. Its lead drug candidate, JAN101, has demonstrated positive data in Phase 2a clinical trials, with Phase 2b trials expected to begin in early 2021 for the treatment and prevention of peripheral artery disease (PAD), a condition that affects over 8.5 million Americans. Other potential indications for JAN101 include diabetic neuropathy, wound healing and vascular inflammation. JanOne continues to operate its legacy businesses under their current brand names, ARCA Recycling and GeoTraq, both of which are undergoing review to determine appropriate strategic alternatives.
Key Growth Drivers
Product Candidate: JanOne acquired an exclusive license to the worldwide right to TV1001SR, now known as JAN101, a twice-daily, orally dosed slow-release formulation. Over $13.5 million1 has been spent on the development in research and clinical work, excluding patent and Intellectual property expenses. Sodium nitrite is an approved drug for acute use and is on the list of The World Health Organizations list of 100 essential medications. Results from Phase 2a clinical trials support the use of sodium nitrite for the treatment and prevention of PAD and as a non-addictive treatment for Diabetic Neuropathy. While not a predetermined endpoint for the trial, subjects who participated in the trial also reported a reduction in pain as a result of the increased blood flow to the extremities and prompted a clinical study into diabetic pain where patients reported a significant reduction in pain.
Market Opportunity: In 2017, the global market for PAD was estimated at nearly $36.1 billion and is expected to grow at a compound annual growth rate (CAGR) of 7.6% to $52.0 billion by 2022, according to BCC Research.2
Patent Portfolio: The company has a portfolio of 30 worldwide patents and other intellectual property relating to sodium nitrite, the sustained release of sodium nitrite, and a provisional application to assist in the treatment of COVID-19 vascular complications. The patent portfolio presents diverse licensing opportunities and potentially royalty opportunities if JanOne intellectual property is used with other drug candidates.
Manufacturing underway: The company has a manufacturing agreement with CoreRx Inc. for the formulation and manufacturing of JAN101. CoreRx operates over 150,000 square feet of cGMP lab and manufacturing facilities, including six formulation suites, 18 manufacturing suites, and two analytical labs.
FDA 505 (b)(2) pathway: To streamline development and approval of the U.S. Food and Drug Administration (the “FDA”), the company expects to pursue FDA 505(b)(2) pathway for new drug approval as a result of an already approved acute use associated with JAN101.
Recent Highlights
|
|
• |
In July 2020, several esteemed colleagues joined JanOne's Scientific Advisory Board |
|
|
• |
In July 2020, JanOne received confirmation from the FDA for the investigational new drug (IND) sponsorship transfer covering its sodium nitrite tablets previously held by Soin Neuroscience |
|
|
• |
In August 2020, JanOne engages CATO-SMS a world leading clinical research organization to assist in the development of JAN101 to Treat COVID-19 Vascular Complications |
|
|
• |
In August 2020, JanOne completes stable formulation of JAN101 in preparation for its first GMP manufacturing batch to support upcoming clinical trials |
|
Market Snapshot—NASDAQ: JAN |
||||
Exhibit 99.1
|
Avg. Vol: 170K |
52-Wk. Range: $2.00-$9.24 |
Shares Outstanding: 2.0M |
Market Cap: $9.7M |
|
|
Price and volume quotes from Yahoo! Finance and other sources - 1Including prior to JanOne’s acquisition of the license - 2https://www.bccresearch.com/market-research/healthcare/the-global-market-for-pain-management-drugs-and-devices.html |
||||
|
JanOne Inc. - 325 E. Warm Springs Road, Suite 102 - Las Vegas, NV 89119 - 800-400-2247 - www.janone.com |
||||
JAN101: A compound with potential broad application
|
|
• |
A safe novel treatment for improving vascular function, reducing neuropathic pain and other conditions resulting from poor blood flow |
|
|
• |
Highly selective, acts only in damaged tissue |
|
|
• |
Very safe, non-addictive and natural product (sodium nitrite) |
|
|
• |
Promotes blood vessel growth and function |
As indicated in multiple human trials, our current sodium nitrite compound has the potential to treat vascular and endothelial cell dysfunction complications experienced by COVID-19 patients.
Multiple phase 2a trials complete
Experienced Management Team
Tony Isaac, CEO – A director of JanOne since May 2015 and CEO since May 2016. Tony Isaac spearheaded the successful turnaround of JanOne and has invested in various companies, both private and public, since 1980.
Tony Giordano, Ph.D., Chief Scientific Officer – Dr. Giordano has held senior management positions at eight biotechnology companies, including four that moved translated drug discovery efforts into early-stage clinical trials.
Amol Soin, MD, Chief Medical Officer – Dr. Soin has been recognized multiple times as one of America's Top Doctors and is the recipient of the Patient's Choice Award, an honor given only to the top 1% of physicians in the country.
Virland Johnson, CFO - was appointed Chief Financial Officer of the Company in 2017. In addition to his role as CFO for JanOne, he also serves as CFO for Live Ventures Incorporated (Nasdaq:LIVE).
Scientific Advisory Board
Chris Kevil, Ph.D., Chair of the Scientific Advisory Board , Edgar Ross, MD, Rakesh Patel, Ph.D., Timothy Ness, MD, Ph.D., Alan Kaye, MD, PhD, DABA, DABPM, DABIPP, John Cooke, MD, Ph.D.
Safe Harbor: This fact sheet contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. In accordance with the safe harbor provisions of this Act, statements contained herein that look forward in time that include everything other than historical information, including statements relating when Phase 2 trials for PAD will begin, involve risks and uncertainties that may affect the company's actual results. These forward-looking statements can be identified by terminology such as "will," "expects," "anticipates," "future," "intends," "plans," "believes," "estimates" and similar statements. JanOne may also make written or oral forward-looking statements in its periodic reports to the U.S. Securities and Exchange Commission (the "SEC") on Forms 10-K and 10-Q, Current Reports on Form 8-K, in its annual report to stockholders, in press releases, and other written materials and in oral statements made by its officers, directors or employees to third parties. There can be no assurance that such statements will prove to be accurate and there are a number of important factors that could cause actual results to differ materially from those expressed in any forward-looking statements made by the company, including, but not limited to, plans and objectives of management for future operations or products, the market acceptance or future success of our products, and our future financial performance. The company cautions that these forward-looking statements are further qualified by other factors including, but not limited to, those set forth in the company's Annual Report on Form 10-K for the fiscal year ended December 28,
Exhibit 99.1
2019 (available at http://www.sec.gov). JanOne undertakes no obligation to publicly update or revise any statements in this release, whether as a result of new information, future events, or otherwise.