

Developing Unique Vascular and Pain Management Medications Summer 2020 Nasdaq: JAN Exhibit 99.1

Disclaimer This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. In accordance with the safe harbor provisions of this Act, statements contained herein that look forward in time that include everything other than historical information, including statements relating to (i) reducing the need for opioids at the prescription pad, (ii) when Phase 2b trials will commence, and (iii) other indications that may benefit from JAN101, including vascular complications resulting from Covid infection. These forward-looking statements can be identified by terminology such as “will,” “aims,” “expects,” “potential,” “anticipates,” “future,” “may,” “intends,” “plans,” “believes,” “estimates” and similar statements. JanOne may also make written or oral forward-looking statements in its periodic reports to the U.S. Securities and Exchange Commission (the “SEC”) on Forms 10-K and 10-Q, Current Reports on Form 8-K, in its annual report to stockholders, in press releases, and other written materials and in oral statements made by its officers, directors or employees to third parties. There can be no assurance that such statements will prove to be accurate and there are a number of important factors that could cause actual results to differ materially from those expressed in any forward-looking statements made by the company, including, but not limited to, plans and objectives of management for future operations or products, the market acceptance or future success of our products, and our future financial performance. The company cautions that these forward-looking statements are further qualified by other factors including, but not limited to, those set forth in the company’s Annual Report on Form 10-K for the fiscal year ended December 28, 2019 (available at http://www.sec.gov). JanOne undertakes no obligation to publicly update or revise any statements in this presentation, whether as a result of new information, future events, or otherwise.

Who is JanOne? Company has exclusively licensed know how and patents after successful pre-clinical, Phase 1 and Phase 2a studies for the biological impact and potential for sodium nitrite to improve pulmonary function A biopharma company whose mission is to develop and introduce drugs into market that will treat conditions that cause severe pain and non-addictive answers to treat pain Ultimately, treating diseases at the source can potentially reduce the need for opioids at the prescription pad Phase 2b trials for the treatment of Peripheral Artery Disease are expected to begin Q1 2021 Listed on NASDAQ: JAN Peripheral Artery Disease, a condition affecting over 60 million people globally, is the company’s initial focus
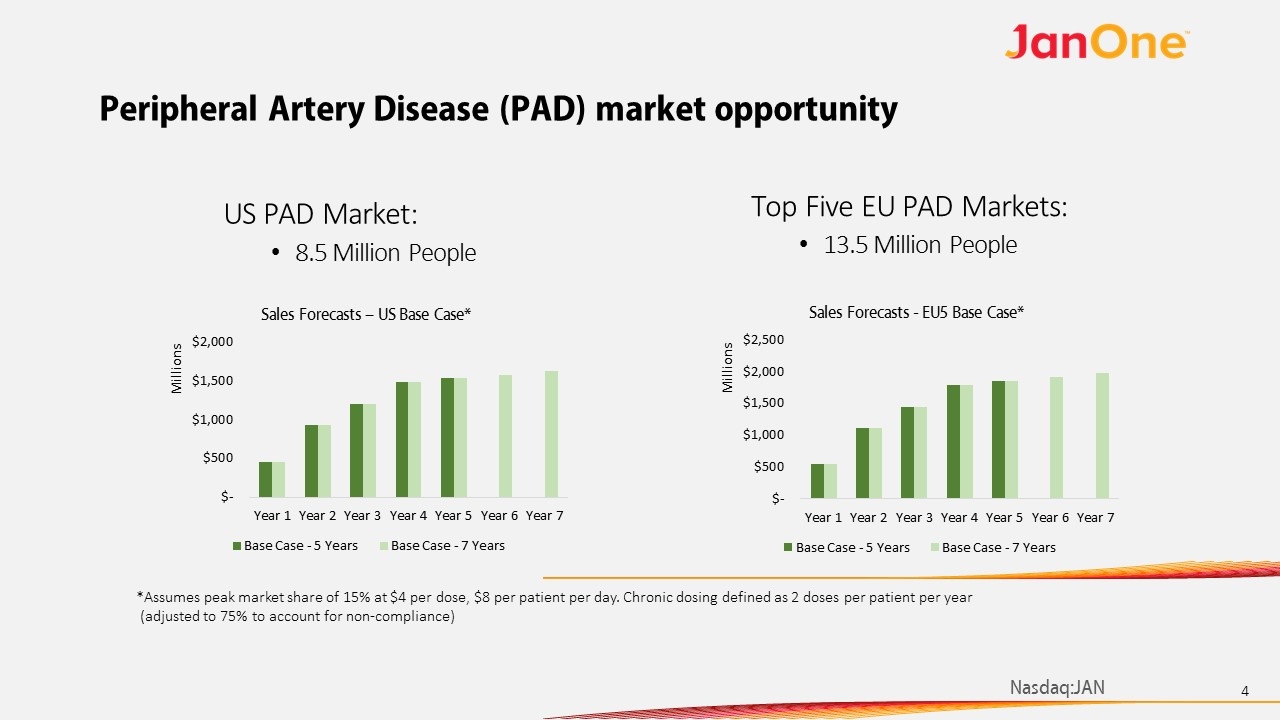
Peripheral Artery Disease (PAD) market opportunity US PAD Market: 8.5 Million People *Assumes peak market share of 15% at $4 per dose, $8 per patient per day. Chronic dosing defined as 2 doses per patient per year (adjusted to 75% to account for non-compliance) Top Five EU PAD Markets: 13.5 Million People
A promising treatment for vascular conditions and pain Sodium nitrite advanced for treating vascular diseases and neuropathic pain JAN101, a sustained release sodium nitrite tablet, to treat PAD - Phase 2b trials expected by Q1 2021 Multiple Phase 2a studies provided evidence of clinical benefit to improve pulmonary function, increase blood flow and reduce associated neuropathic associated pain Over $13 million invested in the development of Jan101* prior to the IND transfer In addition to PAD, other indications that may benefit: Diabetic Neuropathy Wound healing Vascular complications resulting from Covid infection *Does not include IP and patent protection expenses
JAN101: A compound with broad application A safe novel treatment for improving vascular function, reducing neuropathic pain and other conditions resulting from poor blood flow Highly selective, acts only in damaged tissue Very safe, non-addictive and natural product (sodium nitrite) Promotes blood vessel growth and function Prevents tissue inflammation and necrosis Prevents diabetic nephropathy, a leading cause of death in diabetics Significantly reduces pain as observed in three human clinical studies JAN101 Plans to Enter Phase 2b clinical trials for the treatment of PAD
Sustained release formulation (JAN101) In vitro sustained release formulation 30% released in first hour 70% over remaining 7-8 hours Pig study with 80 mg tablets No change in blood pressure (indicator of vasodilation) over 24-hour period after administration Induces markers within 30 days angiogenesis (new blood vessels) Positive trend in angiogenic index after 30 days (new vessel formation) In clinical trial with JAN101 Diabetic neuropathy patients did not report increases in headaches and dizziness
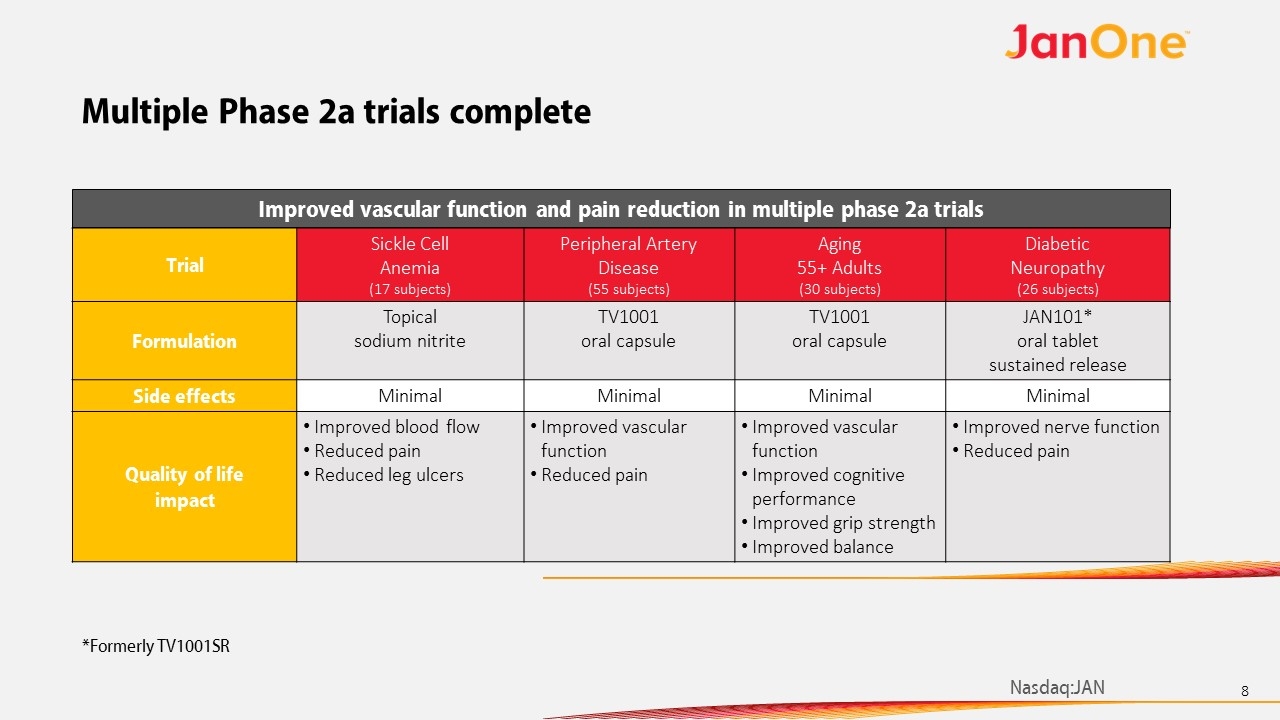
Trial Sickle Cell Anemia (17 subjects) Peripheral Artery Disease (55 subjects) Aging 55+ Adults (30 subjects) Diabetic Neuropathy (26 subjects) Formulation Topical sodium nitrite TV1001 oral capsule TV1001 oral capsule JAN101* oral tablet sustained release Side effects Minimal Minimal Minimal Minimal Quality of life impact Improved blood flow Reduced pain Reduced leg ulcers Improved vascular function Reduced pain Improved vascular function Improved cognitive performance Improved grip strength Improved balance Improved nerve function Reduced pain Improved vascular function and pain reduction in multiple phase 2a trials Multiple Phase 2a trials complete *Formerly TV1001SR
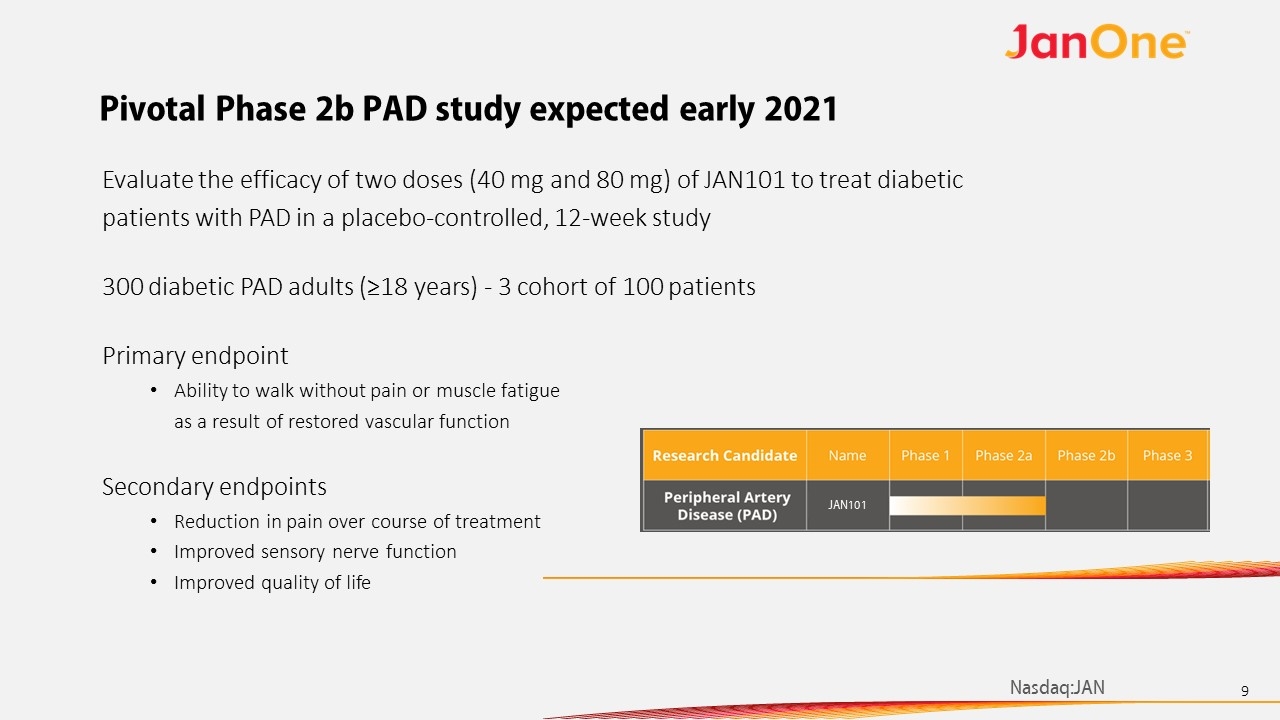
Pivotal Phase 2b PAD study expected early 2021 Evaluate the efficacy of two doses (40 mg and 80 mg) of JAN101 to treat diabetic patients with PAD in a placebo-controlled, 12-week study 300 diabetic PAD adults (≥18 years) - 3 cohort of 100 patients Primary endpoint Ability to walk without pain or muscle fatigue as a result of restored vascular function Secondary endpoints Reduction in pain over course of treatment Improved sensory nerve function Improved quality of life JAN101
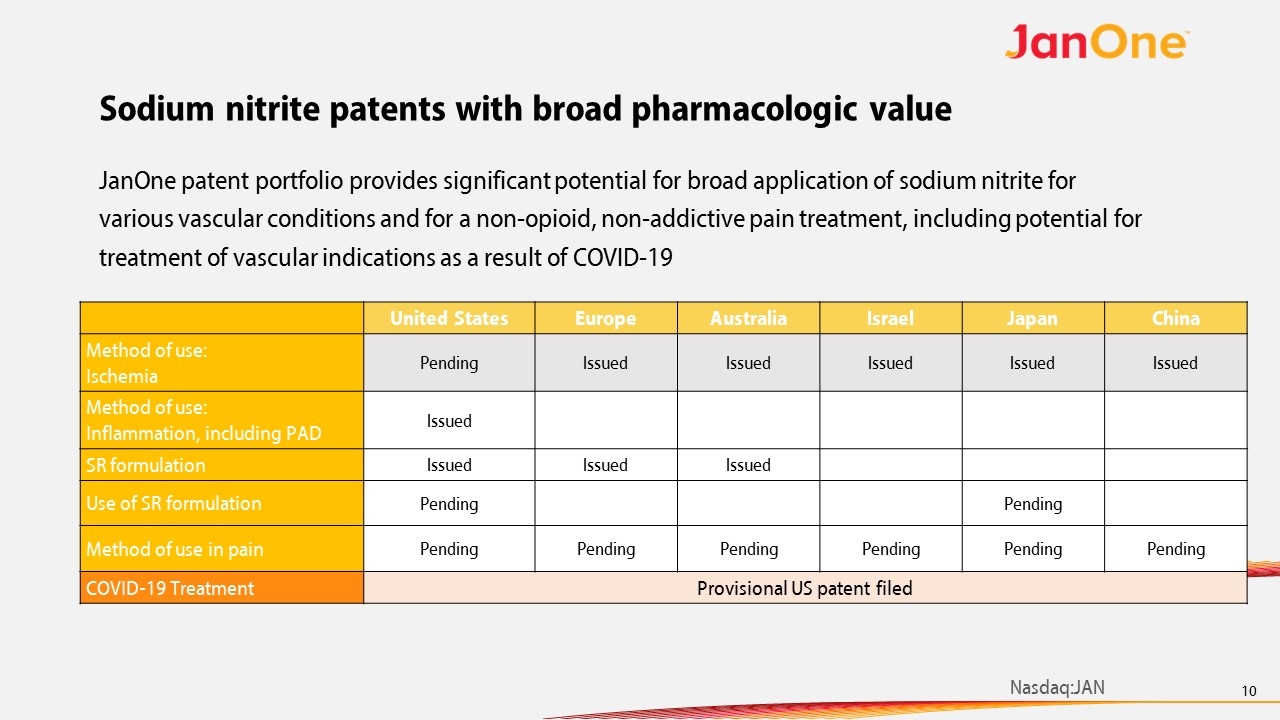
Sodium nitrite patents with broad pharmacologic value United States Europe Australia Israel Japan China Method of use: Ischemia Pending Issued Issued Issued Issued Issued Method of use: Inflammation, including PAD Issued SR formulation Issued Issued Issued Use of SR formulation Pending Pending Method of use in pain Pending Pending Pending Pending Pending Pending COVID-19 Treatment Provisional US patent filed JanOne patent portfolio provides significant potential for broad application of sodium nitrite for various vascular conditions and for a non-opioid, non-addictive pain treatment, including potential for treatment of vascular indications as a result of COVID-19

Emerging opportunity Mounting evidence indicating that COVID-19 may primarily attack the vascular system Endothelial cell dysfunction and inflammation causing severe tissue damage due to lack of oxygen being carried to vital organs, including the lungs, causing respiratory complications

Nature Reviews Immunology In the news Nasdaq:JAN
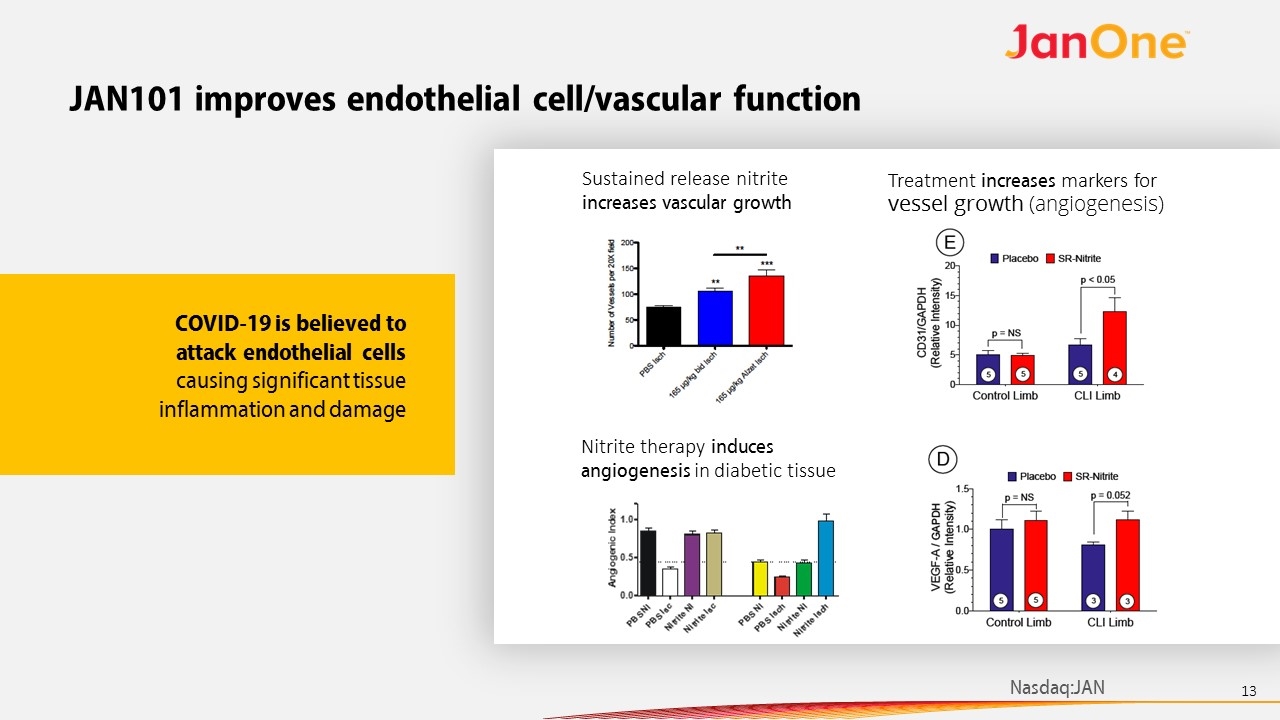
JAN101 improves endothelial cell/vascular function Nitrite therapy induces angiogenesis in diabetic tissue Sustained release nitrite increases vascular growth Treatment increases markers for vessel growth (angiogenesis) COVID-19 is believed to attack endothelial cells causing significant tissue inflammation and damage
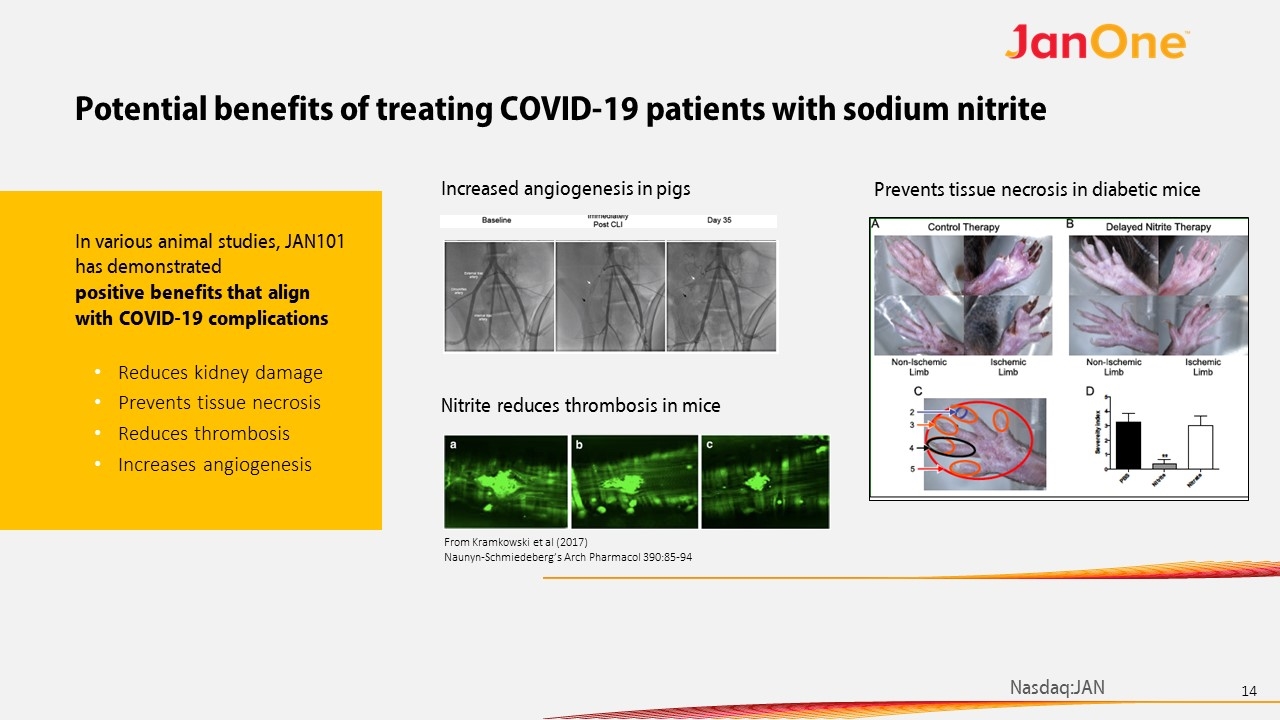
Potential benefits of treating COVID-19 patients with sodium nitrite Increased angiogenesis in pigs Prevents tissue necrosis in diabetic mice From Kramkowski et al (2017) Naunyn-Schmiedeberg’s Arch Pharmacol 390:85-94 Nitrite reduces thrombosis in mice In various animal studies, JAN101 has demonstrated positive benefits that align with COVID-19 complications Reduces kidney damage Prevents tissue necrosis Reduces thrombosis Increases angiogenesis
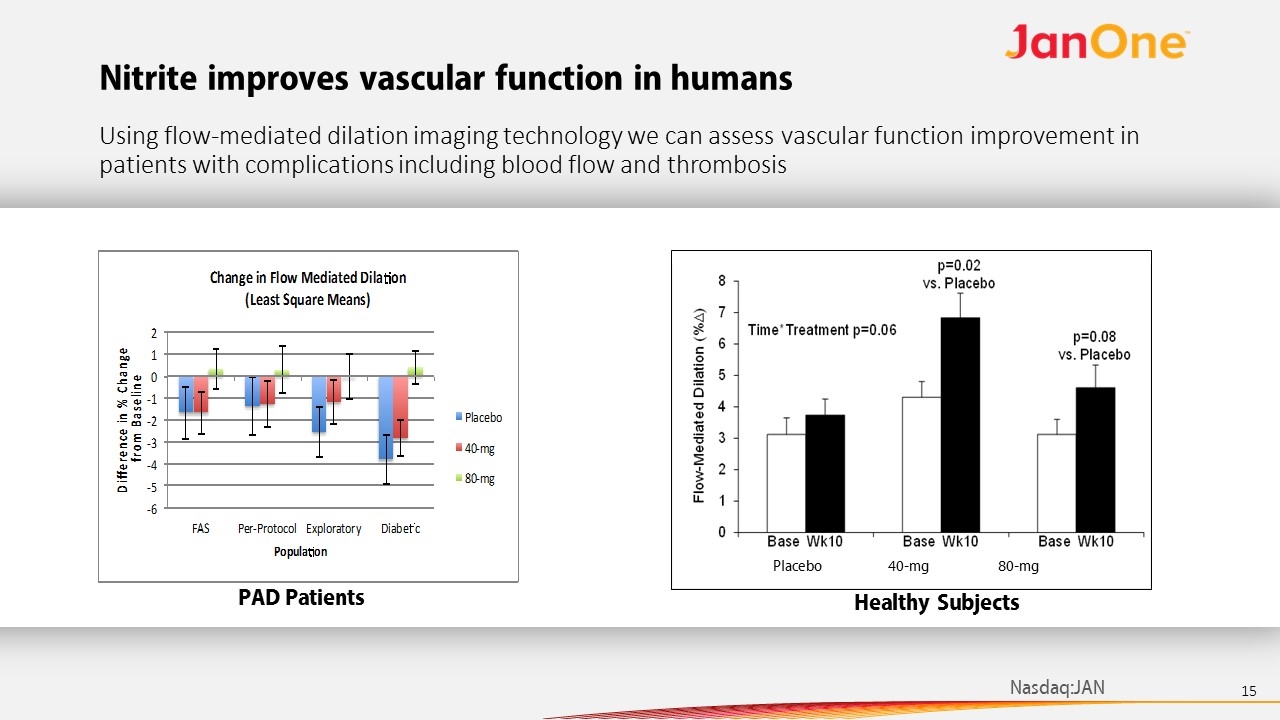
Nitrite improves vascular function in humans Using flow-mediated dilation imaging technology we can assess vascular function improvement in patients with complications including blood flow and thrombosis Healthy Subjects Placebo 40-mg 80-mg PAD Patients
The JAN101 COVID-19 vascular treatment opportunity As indicated in multiple human trials, our current sodium nitrate compound may be a successful treatment for the vascular complications experienced by COVID-19 patients Shown to improve vascular function Shown to reduce vascular complications such as thrombosis Protects major organs from tissue damage due to poor blood flow Inhibits inflammation, including mitigating the “cytokine storm”, a massive release of cytokine proteins that destroy endothelial cells (cells that protect the lining of vessel tissue) Nitrite has proven to be well tolerated and safe Intend to seek emergency use authorization Expanding existing JAN101 IND for clinical study to treat COVID-19 vascular complications

Tony Isaac Chief Executive Officer Tony Giordano, PhD Chief Scientific Officer Virland A. Johnson Chief Financial Officer Michael Stein In-house Counsel and Corporate Secretary Christopher Kevil, PhD Chairman Rakesh Patel, PhD Timothy Ness, MD, PhD Edgar Ross, MD Alan Kaye, MD, PhD, DABA, DABPM, DABIPP John Cooke, MD, PhD Joshua Beckman, MD,MS Management team Amol Soin, MD Chief Medical Officer Scientific advisory board
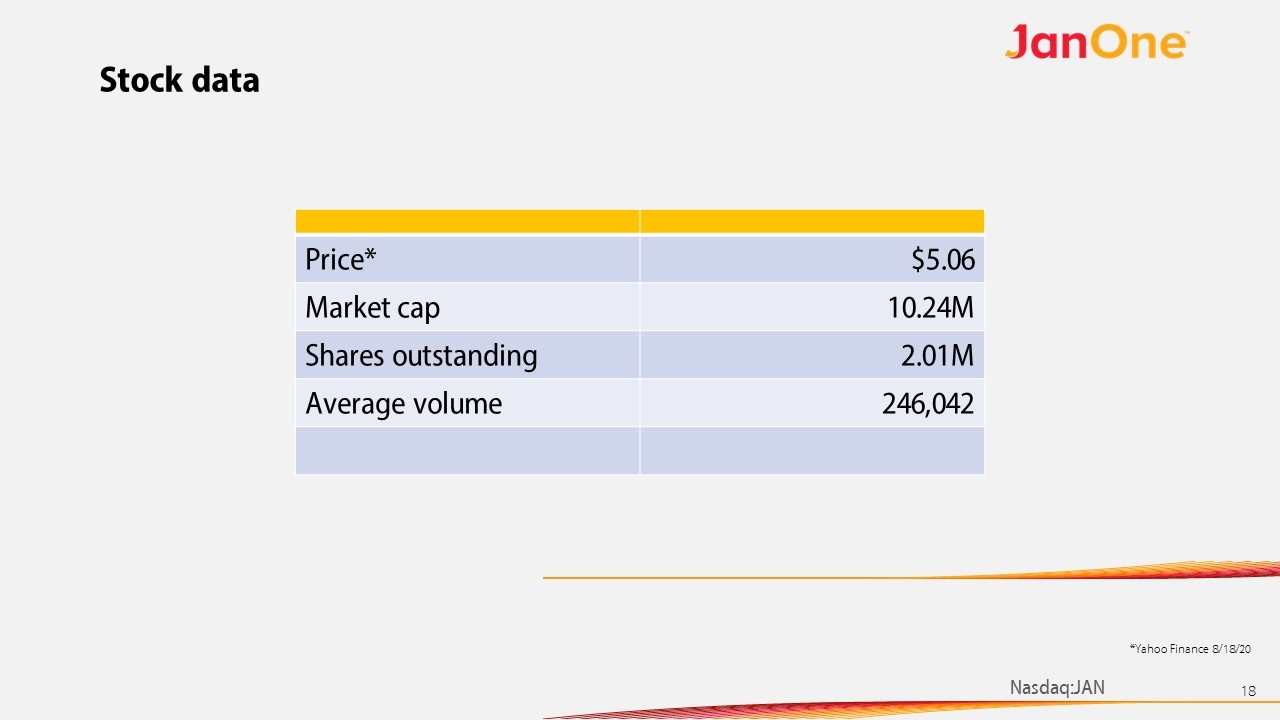
Stock data Price* $5.06 Market cap 10.24M Shares outstanding 2.01M Average volume 246,042 *Yahoo Finance 8/18/20
Evolving solutions for vascular conditions and pain Phase 2b trials for JAN101 for treating PAD expected to start Q1 2021 PAD treatment presents significant domestic and international market opportunity JAN101 is well-tolerated with a strong safety profile JAN101 increases blood flow and reduces vessel inflammation, thus reducing pain Existing sodium nitrite IND can be expanded for other vascular conditions JAN101 therapeutic profile may prove effective for treating COVID-19 vascular complications Partnership and royalty opportunities with strong sodium nitrite patent position Management and scientific advisory board are leading experts in vascular disease and neuropathic pain

Contact: Investor relations 1 (800) 400-2247 ir@janone.com NASDAQ: JAN www.janone.com