
Exhibit 99.1

September 2020
Dear Shareholder:
This past year has been an exciting time for our Company as we have transitioned into what we believe to be a flourishing biopharma company. The initial path forward is to continue the development of our worldwide exclusively licensed, proprietary, high-value, late-stage biopharma asset, JAN101, for the treatment of Peripheral Artery Disease, or PAD. We will also look for new indications for JAN101, like the recently announced potential application for the treatment of vascular complications associated with COVID-19, and novel ways to treat pain without the need for opioids. The biotechnology market demonstrated strong growth throughout most of 2020, with the NASDAQ Biotechnology Index growing by 44% from 2,961 on March 16, 2020, to 4,262 on August 31, 2020.
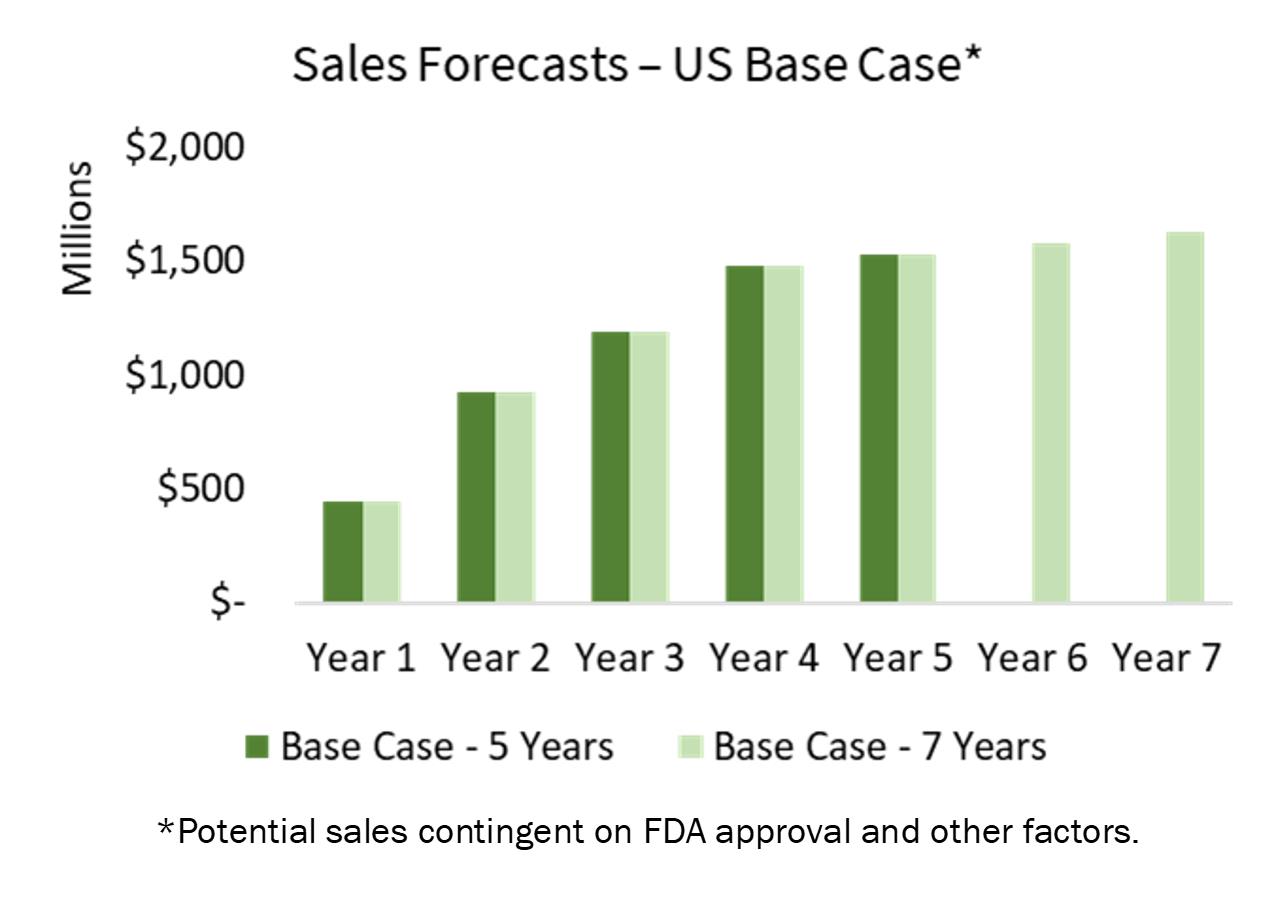 Our core biopharma asset can be applied to three identified and distinct markets that represent significant market opportunities: PAD, pain management, and COVID-19-related therapies. In 2018, the global market size for pain management pharmaceuticals was $38.9 billion and forecasted to reach $52.1 billion by 2024, exhibiting a CAGR of around 5% during 2019-2024.1 Specifically, the PAD market presents significant opportunity with expectations to reach approximately $5 billion by 20232 at a CAGR of 6.8%. Further, according to the CardioVascular Coalition, patients are also at greater risk for heart attack and stroke. Studies have found that the total annual costs for patients with PAD exceed $21 billion, including nearly $10 billion for hospitalizations.3 Most recently, we have determined that our technology could have applications for COVID-19, an area that poses high immediate value to economies across the world. Combined, we believe JanOne is well positioned for success in the application of its patented core formulation component, sodium nitrite, for PAD and potential future product extensions.
Our core biopharma asset can be applied to three identified and distinct markets that represent significant market opportunities: PAD, pain management, and COVID-19-related therapies. In 2018, the global market size for pain management pharmaceuticals was $38.9 billion and forecasted to reach $52.1 billion by 2024, exhibiting a CAGR of around 5% during 2019-2024.1 Specifically, the PAD market presents significant opportunity with expectations to reach approximately $5 billion by 20232 at a CAGR of 6.8%. Further, according to the CardioVascular Coalition, patients are also at greater risk for heart attack and stroke. Studies have found that the total annual costs for patients with PAD exceed $21 billion, including nearly $10 billion for hospitalizations.3 Most recently, we have determined that our technology could have applications for COVID-19, an area that poses high immediate value to economies across the world. Combined, we believe JanOne is well positioned for success in the application of its patented core formulation component, sodium nitrite, for PAD and potential future product extensions.
Over the past year, we have added substantial talent to our team, made progress with finalizing our Phase 2b PAD study protocols for FDA submission, and announced potential applications that address vascular complications relating to COVID-19. Collectively, these accomplishments contribute to a consistent increase in shareholder value and we believe move the company closer to a treatment for PAD, a potential opioid-free, non-addictive pain relief solution and a potential treatment for vascular complications resulting from COVID-19.
|
|
1 |
https://www.prnewswire.com/news-releases/global-52-1bn-pain-management-drugs-market-outlook-2019-2024--300877857.html |
|
2 |
Allied Market Research, March 27, 2019 |
|
3 |
https://cardiovascularcoalition.com /cardiovascular-care/peripheral-artery-disease-pad/ |
1 JanOne Inc.
325 East Warm Springs Road, Suite 102
Las Vegas, NV 89119
www.janone.com
Exhibit 99.1

Recent Highlights
|
|
• |
In January 2020, Dr. Amol Soin, one of the nation’s leading pain experts, joined JanOne as our Chief Medical Officer |
|
|
• |
In February 2020, we signed an agreement with CoreRx to conduct our Phase 2b clinical formulation and development. |
|
|
• |
In June 2020, we announced Dr. Doug Flanagan as Chief Formulation Advisor. |
|
|
• |
In July 2020, the following individuals joined our Scientific Advisory Board: |
▪ Dr. Rakesh Patel, an internationally recognized scientist in nitric oxide and redox biology, known for his work associated with blood flow regulation and pulmonary function.
▪ Dr. Timothy Ness, emeritus professor at University of Alabama at Birmingham, a pain mechanisms and medication expert who has served as a clinical research expert on pain for the National institutes of Health and the Food and Drug Administration.
▪ Dr. Alan Kaye, Chairman of the LSU Health New Orleans Anesthesiology Department and editor in chief of the journal Pain Physicians and who serves on the FDA’s Advisory Board of Anesthetics and Analgesics.
▪ Dr. John Cooke, Chair of the Department of Cardiovascular Sciences at the Houston Methodist Research Institute, who, in his prior role, was recruited to spearhead the program in vascular biology at Stanford University and has served on national and international committees that deal with cardiovascular diseases.
|
|
• |
In July 2020, we received FDA authorization for the transfer of an investigational new drug application (IND) for our sodium nitrite tablets. |
|
|
• |
In August 2020, we completed stable formulation and engineering of JAN101 for GMP (Good Manufacturing Practice) manufacturing to support our planned Phase 2b PAD study. |
|
|
• |
In August 2020, we selected Eurofins Alphora as our bottling and labelling partner to support upcoming clinical trials. |
A Safe Novel Treatment
Our drug candidate, JAN101, a sustained release of sodium nitrite, is a safe and novel treatment for improving vascular function, reducing neuropathic pain and other conditions resulting from poor blood flow. It is highly selective, acting only in damaged tissue. Sodium nitrite in sustained release promotes blood vessel growth and function, prevents tissue inflammation and necrosis, and prevents diabetic nephropathy, a leading cause of death in diabetics. Additionally, three human clinical studies have found that sodium nitrite significantly reduces pain.
Peripheral Artery Disease (PAD)
2 JanOne Inc.
325 East Warm Springs Road, Suite 102
Las Vegas, NV 89119
www.janone.com
Exhibit 99.1

Our first drug candidate, JAN101 (formerly referred to as TV1001SR), is intended to address the 8.5 million Americans who may have PAD. We expect to enter Phase 2b trials in early 2021 after promising results seen in Phase 1 and Phase 2a trials. One of the more encouraging outcomes from patients who participated in early trials was a reported reduction in associated PAD pain. According to a Stanford University research study, up to 24% of patients with PAD are at risk of high opioid use.4 If JAN101 is successful, the potential increase in value to the medical community as a PAD treatment that also relieves associated pain without addictive properties could be significant. The US market opportunity as a PAD treatment that may also treat associated pain without addictive qualities could result in a potential multibillion-dollar revenue stream for JanOne if JAN101 is approved as a new drug by the FDA.
COVID-19
A recent study in the New England Journal of Medicine illustrated that SARS-CoV-2, the virus that causes COVID-19, damages the endothelial cells that line blood vessels that ultimately trigger blood clotting. This restricts and damages the vascular system of those infected with the virus. This illustrates that rather than treating COVID-19 as a respiratory condition, success may be achieved with treating the underlying vascular tissue damage caused by the attack of COVID-19 on endothelial cells.
Based on the New England Journal of Medicine’s research and various other ongoing studies that look at COVID-19 as a vascular disease, the Company’s sodium nitrite compound may prove to be a beneficial treatment for COVID-19. The Company is preparing its IND packages for FDA submission for continued development as a treatment of PAD and to extend JAN101 to potentially mitigate severe organ and tissue damage caused by COVID-19.
Robust Pipeline
The Company has made a strategic decision to initially pursue Peripheral Artery Disease. Our planned pivotal Phase 2b PAD study is scheduled for early 2021. In this study, we will evaluate the efficacy of two doses (40 and 80 mg) of JAN101 to treat diabetic patients with PAD in a placebo-controlled, 12-week study.
Plans are to study 300 diabetic adults with PAD in 3 cohorts of 100 patients each. The primary endpoint will be that the patient has the ability to walk without pain or muscle fatigue as a result of vascular function restored by our drug candidate. The secondary endpoints will be reduction in pain over the course of treatment, improved sensory nerve function and improved quality of life.
Experienced Management Team
One of our greatest assets is the team that has been carefully and strategically assembled to advance JanOne. The decades of experience in business and science of the team provide us with a depth of knowledge and contacts to efficiently and effectively move JAN101 forward and identify and develop new applications.
As a director of JanOne since May 2015 and CEO since May 2016, I have spearheaded the successful turnaround of the company and its transition into biotechnology. I have invested in various companies, both private and public, since 1980. I serve as a director of Live Ventures Incorporated (Nasdaq: LIVE), a holding company for diversified businesses, since December 2011. I am the Chairman and Co-founder of Isaac
|
|
4 |
https://pubmed.ncbi.nlm.nih.gov/30922747/ |
3 JanOne Inc.
325 East Warm Springs Road, Suite 102
Las Vegas, NV 89119
www.janone.com
Exhibit 99.1

Organization. I am a graduate of Ottawa University in 1981, where I majored in Commerce and Business Administration and Economics.
Tony Giordano, Ph.D., Chief Scientific Officer – Dr. Giordano has held senior management positions at eight biotechnology companies, including four that moved drug discovery efforts into early-stage clinical trials. As CEO of TheraVasc, he was responsible for the development of TV1001 our leading asset now known as JAN101, Most recently, he served as Senior Director of Special Projects in the Innovations group at the Cleveland Clinic, an American academic medical center ranked #2 as best hospital in the world by U.S. News & World Report. Dr. Giordano has secured over $6M in federal funding for companies he has managed. He previously held positions as a senior scientist at Abbott Laboratories, Staff Fellow at NIA, and Biotech Fellow at NCI.
Amol Soin, MD, Chief Medical Officer – Dr. Soin has been recognized as one of America’s Top Doctors and is the recipient of the Patient's Choice Award, an honor given only to the top 1% of physicians in the country. Dr. Soin is founder of The Ohio Pain Clinic, a network of free-standing chronic pain management facilities in southwestern Ohio focused on non-opioid-based treatments for chronic pain. Dr. Soin was appointed by Governor Kasich to the Ohio Medical Board in 2012 to two 5-year terms and has served as the Ohio Medical Board’s president, during which time he was instrumental in passing statewide rules and guidelines to help the opioid crisis. Dr. Soin is a nationally recognized speaker and author of the book Curing America. He has authored a range of research papers, publications, and abstracts and served as a primary investigator for various clinical research initiatives. Presently, Dr. Soin serves as the State of Ohio’s Medicare Care Advisory Committee’s Pain Management Representative and serves on the prescription drug abuse committee and legislative task force for the Ohio State Medical Association.
Doug Flanagan, Ph.D., Chief Formulation Advisor -- Dr. Flanagan was engaged by JanOne to work on its proprietary sustained release formulation, and he currently works closely with CoreRx to ensure chemical formulation compliance. He brings us over 40 years of scientific, pharmaceutical formulation and clinical experience and has published over 90 peer-reviewed articles and delivered over 150 presentations, talks and lectures around the world. He has lent his formulation expertise to over 50 pharmaceutical companies and medical organizations, including Abbott Laboratories, Pfizer Animal Health (spun off as Zoetis), Bristol Myers Squibb, the World Health Organization and the Food and Drug Administration, to name a few. Dr. Flanagan also holds various formulation patents, including pharmaceutical formulations of nitrite, a key component of JAN101. Dr. Flanagan was appointed as Emeritus Professor at the University of Iowa College of Pharmacy, where he served as a faculty member for 34 years.
Michael Stein, Legal Counsel and Corporate Secretary – Mr. Stein joined the company in October 2017. Mr. Stein also serves as Senior Vice President and General Counsel for Live Ventures Incorporated (Nasdaq: LIVE). Prior to joining JanOne, Mr. Stein was a corporate partner at DLA Piper LLP from April 2016 through October 2017, and an associate from 2005 through 2012, where he gained biotechnology transaction experience. Prior to rejoining DLA, Mr. Stein worked at Caesars Entertainment Corporation (Nasdaq: CZR) and at Everi Holdings Inc. (NYSE: EVRI). Mr. Stein holds a JD from the University of Maryland, and an MS and BS in Accounting from the University of Florida.
Virland Johnson, CFO – Mr. Johnson was appointed Chief Financial Officer of the Company in 2017. In addition to his role as CFO for JanOne, he also serves as CFO for Live Ventures Incorporated (Nasdaq: LIVE). Prior to joining JanOne, Mr. Johnson was Senior Director of Revenue for JDA Software from February 2010 to April 2016, where he was responsible for revenue recognition determination and sales and contract support while acting as a
4 JanOne Inc.
325 East Warm Springs Road, Suite 102
Las Vegas, NV 89119
www.janone.com
Exhibit 99.1

subject matter expert. Prior to joining JDA, Mr. Johnson provided leadership and strategic direction while serving in C-level executive roles in public and private companies such as Cultural Experiences Abroad, Inc., Fender Musical Instruments Corp., Triumph Group, Inc., Unitech Industries, Inc. and Younger Brothers Group, Inc. Mr. Johnson’s more than 25 years of experience is primarily in SEC and financial reporting. Mr. Johnson holds a bachelor’s degree in Accountancy from Arizona State University.
Scientific Advisory Board
Chris Kevil, Ph.D., Chair of the Scientific Advisory Board – Dr. Kevil, an internationally known expert in vascular pathophysiology, PAD, and nitric oxide biology, discovered the role of sodium nitrite in promoting angiogenesis that led to the development of TV1001, now known as JAN101. Dr. Kevil earned his Ph.D. from LSU Health Shreveport in Molecular and Cellular Physiology, followed by a fellowship at the University of Alabama at Birmingham (UAB) with an emphasis on redox pathophysiology. Returning to LSU Health Shreveport in the Department of Pathology, he established cutting-edge research programs regarding redox biology regulation of peripheral vascular diseases. This led to groundbreaking insights on how glutathione, nitrite/nitric oxide, and hydrogen sulfide regulate vascular health during ischemia.
Edgar Ross, MD – Dr. Ross is the current Director of the Pain Management Center at Brigham and Women’s Hospital and a professor of anesthesia at Harvard Medical School. Dr. Ross is recognized as one of Castle Connolly Top Doctors for the fifth year in a row. In addition to serving as chairman of Pfizer’s partnership on pain, Dr. Ross also has served as a member of the Blue Cross and Blue Shield Opioid Prescribing Policy Committee.
Rakesh Patel, Ph.D. – Dr. Patel is currently Vice Chair for Research, Department of Pathology, and Director of the Center for Free Radical Biology at the University of Alabama at Birmingham (UAB). Most noted is his research to understand the molecular basis of nitric oxide, and nitrite interactions with organs and red blood cells. Dr. Patel is also known for his work to understand the impacts on the biological process associated with blood flow regulation and pulmonary function.
Timothy Ness, MD, Ph.D. – Dr. Ness is Professor Emeritus and former Pain Treatment Division Chief, Director of Pain Research and Vice Chair for Clinical Research in the Department of Anesthesiology and Perioperative Medicine at the University of Alabama at Birmingham (UAB). He has served as a clinical research expert on pain for the National Institutes of Health (NIH), Food and Drug Administration (FDA) advisory panels, the Veterans Administration (VA), and various international research institutes. He has served on the American Pain Society and the American Society of Regional Anesthesia and Pain Medicine Board of Directors. He is currently funded by the NIH.
Alan Kaye, MD, Ph.D., DABA, DABPM, DABIPP – Dr. Kaye has been the Professor and Chairman of the Department of Anesthesiology at LSU Health Sciences Center in New Orleans since January 2005. Before that, he was Professor and Chairman of the Texas Tech University Health Sciences Center’s Department of Anesthesiology in Lubbock, Texas. Prior to that, he was the Medical Director of the Greater New Orleans Surgical Center, the Director of Resident Recruitment, Acting Program Director and an Attending Staff of the Department of Anesthesiology at Tulane University Medical Center in New Orleans. He received two BS degrees and a MD degree from the University of Arizona. He also completed a pain management fellowship at Texas Tech Health
5 JanOne Inc.
325 East Warm Springs Road, Suite 102
Las Vegas, NV 89119
www.janone.com
Exhibit 99.1

Sciences Center. He is board certified as a Consultant in Anesthesiology and has a special certificate in Pain Management for the American Board of Anesthesiology. He is also a Diplomate of the American Board of Pain Medicine and the American Board of Interventional Pain Physicians. He has authored or co-authored over 150 abstracts and 200 manuscripts and book chapters in the fields of pulmonary vascular pharmacology and anesthesiology. He serves on a number of national committees, including the National Board of Directors of ASIPP and ABIPP. He is editor in chief of the journal Pain Physicians and is on the FDA Advisory Board on Anesthetics and Analgesics. He was an Associate National Board Examiner in Anesthesiology.
John Cooke, MD, Ph.D. – Dr. Cooke is the Chair of the Department of Cardiovascular Sciences at the Houston Methodist Research Institute, Director of the Center for Cardiovascular Regeneration, and Medical Director of the RNA Therapeutics Program in the Houston Methodist DeBakey Heart and Vascular Center in Houston, Texas. He trained in cardiovascular medicine and obtained a Ph.D. in physiology at the Mayo Clinic. He was recruited to Harvard Medical School as an assistant professor of medicine. In 1990, he was recruited to Stanford University to spearhead the program in vascular biology and medicine, and he was appointed professor in the Division of Cardiovascular Medicine at Stanford University School of Medicine, and associate director of the Stanford Cardiovascular Institute until his recruitment to Houston Methodist in 2013. Dr. Cooke has published over 500 research papers, position papers, reviews, book chapters and patents in the arena of vascular medicine and biology, with over 30,000 citations. He has served on national and international committees that deal with cardiovascular diseases, including the American Heart Association, American College of Cardiology, Society for Vascular Medicine, and the National Heart, Lung, and Blood Institute. He has served as president of the Society for Vascular Medicine, as a director of the American Board of Vascular Medicine, and as an associate editor of Vascular Medicine.
A Bright Future Ahead
We feel strongly that the targeted indication areas of potentially treating PAD, pain management and treating vascular complications of COVID-19 represent promising treatment areas for our proprietary drug platform. We believe that the pivotal Phase 2b study for PAD and the IND submission for the COVID-19 protocol are compelling upcoming catalysts for the advancement of our high-value late-stage biopharma asset. We have assembled a world-class team of scientific and clinical experts in vascular disease and pain management that are leading the future success of our Company. We believe the continued development of our intellectual property and the potential value that JanOne brings to patients will gain recognition from investors and the medical community alike.
Thank you for your confidence in our Company, and we look forward to making a difference in millions of lives in the future.
Sincerely,
Tony Isaac
Chief Executive Officer
6 JanOne Inc.
325 East Warm Springs Road, Suite 102
Las Vegas, NV 89119
www.janone.com
Exhibit 99.1

Forward-Looking and Cautionary Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. In accordance with the safe harbor provisions of this Act, statements contained herein that look forward in time that include everything other than historical information, including statements relating to (i) whether JAN101 can treat vascular complications in COVID-19 patients, (ii) whether the company can obtain FDA approval for its COVID-19 study, (iii) when the Phase 2b trials for PAD commence, and (iv) when and whether the company will submit an IND for the treatment of COVID-19 vascular complications, and (v) when manufacturing of JAN101 will commence. These forward-looking statements can be identified by terminology such as “will,” “aims,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates” and similar statements. JanOne may also make written or oral forward-looking statements in its periodic reports to the U.S. Securities and Exchange Commission (the “SEC”) on Forms 10-K and 10-Q, Current Reports on Form 8-K, in its annual report to stockholders, in press releases, and other written materials and in oral statements made by its officers, directors or employees to third parties. There can be no assurance that such statements will prove to be accurate, and there are a number of important factors that could cause actual results to differ materially from those expressed in any forward-looking statements made by the company, including, but not limited to, plans and objectives of management for future operations or products, the market acceptance or future success of our products, and our future financial performance. The company cautions that these forward-looking statements are further qualified by other factors including, but not limited to, those set forth in the company’s Annual Report on Form 10-K for the fiscal year ended December 28, 2019 (available at http://www.sec.gov). JanOne undertakes no obligation to publicly update or revise any statements in this release, whether as a result of new information, future events, or otherwise.
7 JanOne Inc.
325 East Warm Springs Road, Suite 102
Las Vegas, NV 89119
www.janone.com