
As filed with the Securities and Exchange Commission on December 23, 2020
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-3
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
JANONE INC.
(Exact name of registrant as specified in its charter)
|
Nevada |
|
5700 |
|
41-1454591 |
|
(State or other jurisdiction of incorporation or organization) |
|
(Primary Standard Industrial Classification Code Number) |
|
(I.R.S. Employer Identification Number) |
325 E. Warm Springs Road, Suite 102
Las Vegas, Nevada 89119
(702) 997-5968
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Tony Isaac
President and Chief Executive Officer
JanOne Inc.
325 E. Warm Springs Road, Suite 102
Las Vegas, Nevada 89119
(702) 997-5968
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With Copy to:
Randolf W. Katz, Esq.
Clark Hill LLP
1055 West Seventh Street, Suite 2400
Los Angeles, California 90017
213-891-9100
Approximate date of commencement of proposed sale to the public:
From time to time after this registration statement is declared effective.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. ☐
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
|
|
|
|
|
Emerging growth company |
|
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of Securities Act. ☐
Calculation of Registration Fee:
|
Title of each class of securities to be registered(1) |
Amount to be registered(2)(3) |
Proposed maximum offering price per security(2)(3)(4)(5) |
Aggregate maximum offering price(2)(3)(4)(5) |
Amount of registration fee(6) |
|
Common Stock, par value $0.001 per share |
|
|
|
|
|
Preferred Stock, par value $0.001 per share |
|
|
|
|
|
Debt Securities |
|
|
|
|
|
Warrants |
|
|
|
|
|
Rights |
|
|
|
|
|
Units(7) |
|
|
|
|
|
Total |
|
|
$100,000,000 |
$ 10,910 |
|
(1) |
Securities registered hereunder may be sold separately, together, or as units with other securities registered hereunder. |
|
(2) |
The proposed maximum aggregate offering price per class of security will be determined from time to time by the registrant in connection with the issuance by the registrant of the securities registered hereunder and is not specified as to each class of securities pursuant to Form S-3 General Instruction II.D. |
|
(3) |
The registrant is registering an indeterminate aggregate principal amount and number of securities of each identified class of securities up to a proposed aggregate offering price of $100,000,000, which may be offered from time to time in unspecified numbers and at indeterminate prices, and as may be issuable upon exercise of any securities registered hereunder, including under any applicable anti-dilution provisions. In addition, pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), the securities being registered hereunder includes such indeterminate number of shares of common stock as may be issuable with respect to the shares being registered hereunder as a result of stock splits, stock dividends, or similar transactions. In no event will the aggregate offering price of all securities issued by the registrant from time to time pursuant to this registration statement exceed $100,000,000, excluding accrued interest, if any, on any debt securities issued under this registration statement. |
|
(4) |
Pursuant to General Instruction II.D. of Form S-3, the table lists each of the classes of securities being registered and the aggregate proceeds to be raised, but does not specify by each class information as to the amount to be registered, proposed maximum offering price per unit, and proposed maximum aggregate offering price. |
|
(5) |
Includes consideration to be received by us, if applicable, for registered securities that are issuable upon exercise, conversion, or exchange of other registered securities. |
|
(6) |
The proposed maximum aggregate offering price has been estimated solely to calculate the registration fee in accordance with Rule 457(o) under the Securities Act. The Registrant previously paid this amount in connection with the filing of the Registration Statement on Form S-3 (File No. 333-248914), which has subsequently been withdrawn, and under Rule 457(p) applies such amount to this registration statement. |
|
(7) |
Each Unit consists of any combination of two or more of the securities being registered hereby. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information contained in this prospectus is not complete and may be changed. We may not sell these securities until the Registration Statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not a solicitation of an offer to buy these securities in any jurisdiction where such offer or sale is not permitted.
Preliminary Prospectus Subject to completion, Dated December 23, 2020

$100,000,000
Common Stock
Preferred Stock
Debt Securities
Warrants
Rights
Units
We may offer and sell from time to time shares of our common stock, par value $0.001 per share (our “Common Stock”), shares of our preferred stock, par value $0.001 per share (our “Preferred Stock”), debt securities, warrants, rights, and units that include any of these securities. The Preferred Stock or warrants may be convertible into or exercisable for shares of our Common Stock or shares of our Preferred Stock or other of our securities registered hereunder. The debt securities may be convertible into or exchangeable for shares of our Common Stock or shares of our Preferred Stock. Our Common Stock is listed on The Nasdaq Capital Market and trades under the symbol “JAN.”
We may offer and sell these securities to or through one or more underwriters, dealers, and agents, or directly to purchasers, on a continuous or delayed basis.
The aggregate market value of our outstanding Common Stock held by non-affiliates was approximately $2,186,586, based on 1,829,982 shares of outstanding Common Stock as of December 15, 2020, of which approximately 410,121 shares were held by affiliates, and based on the closing sale price of our Common Stock of $4.62 on November 25, 2020. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities pursuant to this prospectus with a value of more than one-third of the aggregate market value of our Common Stock held by non-affiliates in any 12-month period, so long as the aggregate market value of our Common Stock held by non-affiliates is less than $75,000,000. In the event that, subsequent to the date of this prospectus, the aggregate market value of our outstanding Common Stock held by non-affiliates equals or exceeds $75,000,000, then the one-third limitation on sales shall not apply to additional sales made pursuant to this prospectus. During the prior 12 calendar months prior to, and including, the date of this prospectus, we have not sold any securities pursuant to General Instruction I.B.6 of Form S-3.
This prospectus describes some of the general terms that may apply to these securities and the general manner in which they may be offered. The specific terms of any securities to be offered, and the specific manner in which they may be offered, will be described in a supplement to this prospectus. You should read this prospectus and any applicable prospectus supplement carefully before you invest.
See the “Risk Factors” section of this prospectus on page 4, our filings with the SEC, and the applicable prospectus supplement for certain risks that you should consider before investing in our securities.
None of the Securities and Exchange Commission, any state securities commission, or any other regulatory body has approved or disapproved of these securities nor passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2020.
TABLE OF CONTENTS
|
|
Page |
|
1 |
|
|
2 |
|
|
3 |
|
|
4 |
|
|
4 |
|
|
5 |
|
|
42 |
|
|
43 |
|
|
44 |
|
|
48 |
|
|
49 |
|
|
59 |
|
|
62 |
|
|
63 |
|
|
63 |
|
|
64 |
|
|
66 |
|
|
66 |
This document is called a prospectus and is part of a Registration Statement on Form S-3 that we have filed with the Securities and Exchange Commission (the “SEC”) using a “shelf” registration process. Under this shelf registration process, we may, from time to time, sell any combination of the securities described in this prospectus in one or more offerings in amounts that we will determine from time to time, up to a total dollar amount of $100,000,000.
This prospectus provides you with a general description of the securities we may offer. Each time we offer a type or series of securities described in this prospectus we will provide a prospectus supplement, incorporate information or document by reference into this prospectus or a related free writing prospectus or use other offering materials, as applicable, containing more specific information about the terms of the securities that are then being offered. We may also authorize one or more related free writing prospectuses to be provided to you that may contain material information relating to these offerings and securities. This prospectus, together with applicable prospectus supplements, any information or document incorporated by reference, and any related free writing prospectus or other offering materials, as applicable, we file with the SEC, includes all material information relating to these offerings and securities. We may also add, update, or change in the prospectus supplement any of the information contained in this prospectus or in the documents that we incorporate by reference into this prospectus, including, without limitation, a discussion of any risk factors or other special considerations that apply to these offerings or securities or the specific plan of distribution. If there is any inconsistency between the information in this prospectus and a prospectus supplement or information or document incorporated by reference having a later date, you should rely on the information in that prospectus supplement or incorporated information having a later date. We urge you to read carefully this prospectus, any applicable prospectus supplement, and any related free writing prospectus or other offering materials, as applicable, together with the information incorporated herein by reference as described under the heading “Incorporation of Certain Information by Reference,” before buying any of the securities being offered.
You should rely only on the information we have provided in, or incorporated by reference into, this prospectus, any applicable prospectus supplement, and any related free writing prospectus or other offering materials, as applicable. We have not authorized anyone to provide you with different information. No dealer, salesperson, or other person is authorized to give any information or to represent anything not contained in this prospectus, any applicable prospectus supplement, any related free writing prospectus, or other offering materials, as applicable.
Neither the delivery of this prospectus nor any sale made under it implies that there has not been any change in our business or affairs or that the information in this prospectus is correct as of any date after the date of this prospectus. You should assume that the information in this prospectus, any applicable prospectus supplement, any related free writing prospectus, or other offering materials, as applicable, is accurate only as of the date on the front of the document and that any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus, any applicable prospectus supplement, any related free writing prospectus, or other offering materials, as applicable, or any sale of a security.
The Registration Statement containing this prospectus, including exhibits to the Registration Statement, provides additional information about us and the securities offered under this prospectus and any prospectus supplement. We have filed and plan to continue to file other documents with the SEC that contain information about us and our business. Also, we will file legal documents that control the terms of the securities offered by this prospectus as exhibits to the reports that we file with the SEC. The Registration Statement and other reports can be read at the SEC Internet site or at the SEC offices mentioned under the heading “Available Information.”
This prospectus contains summaries of certain provisions contained in some of the documents described herein; but, reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed, or will be incorporated by reference as exhibits to the Registration Statement of which this prospectus is a part, and you may obtain copies of those documents as described below under “Available Information.”
1
We have filed with the SEC a Registration Statement on Form S-3 under the Securities Act with respect to the securities covered by this prospectus. This prospectus, which is a part of that Registration Statement, does not contain all of the information set forth in the Registration Statement or the exhibits and schedules filed therewith. For further information with respect to us and the securities covered by this prospectus, please see the Registration Statement and the exhibits filed with the Registration Statement. A copy of the Registration Statement and the exhibits filed with the Registration Statement may be inspected without charge at the Public Reference Room maintained by the SEC, located at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the Public Reference Room. The SEC also maintains an Internet website that contains reports, proxy and information statements, and other information regarding registrants that file electronically with the SEC. The address of the website is http://www.sec.gov.
We are subject to the information and periodic reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and, in accordance therewith, we file periodic reports, proxy statements, and other information with the SEC. Such periodic reports, proxy statements, and other information are available for inspection and copying at the Public Reference Room and website of the SEC referred to above. We maintain a website at http://www.janone.com. You may access our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed pursuant to Sections 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. Our website and the information contained on that site, or connected to that site, are not incorporated into and are not a part of this prospectus.
2
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC’s rules allow us to incorporate by reference information into this prospectus. This means that we can disclose important information to you by referring you to another document. Any information referred to in this way is considered part of this prospectus from the date we file that document. Any reports filed by us with the SEC after the date of this prospectus and before the date that the offering of the securities by means of this prospectus is terminated will automatically update and, where applicable, supersede any information contained in this prospectus or incorporated by reference in this prospectus.
We incorporate by reference into this prospectus the following documents or information filed with the SEC (other than, in each case, documents or information deemed to have been furnished and not filed in accordance with SEC rules):
|
• |
Our Annual Report on Form 10-K for the year ended December 28, 2019, filed with the SEC on April 6, 2020; |
|
• |
Our Quarterly Reports on Form 10-Q for the quarters ended March 28, 2020, filed with the SEC on May 12, 2020, June 27, 2020, filed with the SEC on August 10, 2020, and September 26, 2020, filed with the SEC on November 10, 2020; |
|
• |
Our Current Reports on Form 8-K, filed with the SEC on January 10, 2020, April 22, 2020 (as amended on April 23, 2020), May 4, 2020, June 18, 2020, June 25, 2020, June 30, 2020, July 8, 2020, July 21, 2020, July 30, 2020, August 6, 2020, August 12, 2020, September 3, 2020, September 16, 2020, September 24, 2020, and October 2, 2020 (excluding any information furnished pursuant to Item 2.02 or Item 7.01 of such Current Reports on Form 8-K); and |
|
• |
The description of our Common Stock contained filed as Exhibit 4.1 to our Annual Report on Form 10-K for the year ended December 28, 2019, filed with the SEC on April 6, 2020. |
Additionally, all documents filed by us with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act, after (i) the date of the initial Registration Statement and prior to effectiveness of the Registration Statement and (ii) the date of this prospectus and before the termination or completion of this offering, shall be deemed to be incorporated by reference into this prospectus from the respective dates of filing of such documents, except that we do not incorporate any document or portion of a document that is “furnished” to the SEC, but not deemed “filed.” Any information that we subsequently file with the SEC that is incorporated by reference as described above will automatically update and supersede any previous information that is part of this prospectus.
We will provide without charge to each person, including any beneficial owner, to whom this prospectus is delivered, upon his or her written or oral request, a copy of any or all documents referred to above that have been or may be incorporated by reference into this prospectus, excluding exhibits to those documents unless they are specifically incorporated by reference into those documents. Written or telephone requests should be directed to JanOne Inc., 325 E. Warm Springs Road, Suite 102, Las Vegas, Nevada 89119, Attention: Corporate Secretary; telephone: (702) 997-5968.
3
This prospectus, including the documents we incorporate by reference into it, contains forward-looking statements within the meaning of Section 27A of the Securities Act, and Section 21E of the Exchange Act, the Private Securities Litigation Reform Act of 1995 (the “PSLRA”) or in releases made by the SEC. Such statements include, without limitation, statements regarding our expectations, hopes, or intentions regarding the future. Statements that are not historical fact are forward-looking statements. These forward looking statements can often be identified by their use of words such as “expect,” “believe,” “anticipate,” “outlook,” “could,” “target,” “project,” “intend,” “plan,” “seek,” “estimate,” “should,” “will,” “may,” and “assume,” as well as variations of such words and similar expressions referring to the future. These cautionary statements are being made pursuant to the Securities Act, the Exchange Act, and the PSLRA with the intention of obtaining the benefits of the “safe harbor” provisions of such laws.
The forward-looking statements contained in or incorporated by reference into this prospectus are largely based on our expectations, which reflect estimates and assumptions made by our management. These estimates and assumptions reflect our best judgment based on currently known market conditions and other factors. Although we believe such estimates and assumptions to be reasonable, they are inherently uncertain and involve certain risks and uncertainties, many of which are beyond our control. If any of those risks and uncertainties materialize, actual results could differ materially from those discussed in any such forward-looking statement. Among the factors that could cause actual results to differ materially from those discussed in forward-looking statements are those discussed under the heading “Risk Factors” below, those discussed under the heading “Risk Factors” and in other sections of our Annual Report on Form 10-K for the year ended December 28, 2019, as well as in our other reports filed from time to time with the SEC that are incorporated by reference into this prospectus. See “Available Information” and “Incorporation of Certain Information by Reference” for information about how to obtain copies of those documents.
All readers are cautioned that the forward-looking statements contained in this prospectus and in the documents incorporated by reference into this prospectus are not guarantees of future performance, and we cannot assure any reader that such statements will be realized or that the forward-looking events and circumstances will occur. Actual results may differ materially from those anticipated or implied in the forward-looking statements. All forward-looking statements in this prospectus and the documents incorporated by reference into it are made only as of the date of the document in which they are contained, based on information available to us as of the date of that document, and we caution you not to place undue reliance on forward-looking statements in light of the risks and uncertainties associated with them. Except as required by law, we undertake no obligation to update any forward-looking statements, whether as a result of new information, future events, or otherwise.
Investing in our securities involves significant risks. You should review carefully the risks and uncertainties described under the heading “Risk Factors” contained in, or incorporated into, the applicable prospectus supplement, any related free writing prospectus, or other offering materials, as applicable, and under similar headings in the other documents that are incorporated by reference herein or therein. Each of the referenced risks and uncertainties could adversely affect our business, operating results, and financial condition, as well as adversely affect the value of an investment in our securities. When we offer and sell any securities pursuant to a prospectus supplement, we may include additional risk factors relevant to such securities in the prospectus supplement.
4
General
As of September 10, 2019, JanOne Inc. (formerly known as Appliance Recycling Centers of America, Inc.) and subsidiaries (collectively, “we,” the “Company,” or “JanOne”) broadened its business perspectives to being a pharmaceutical company focused on finding treatments for conditions that cause severe pain and bringing to market drugs with non-addictive pain-relieving properties. The Company aims to reduce prescriptions for dangerous opioid drugs by treating underlying diseases that cause severe pain. Our first drug candidate is a treatment for Peripheral Arterial Disease (“PAD”), a condition that can cause severe pain and affects over 8.5 million people in the U.S. alone. In addition, we continue to operate our legacy businesses, ARCA Recycling, Inc. (“ARCA Recycling”), in our Recycling segment, and GeoTraq Inc. (“GeoTraq”), in our Technology segment. ARCA Recycling recycles major household appliances in North America by providing turnkey appliance recycling and replacement services for utilities and other sponsors of energy efficiency programs. GeoTraq is engaged in the development, design, and, ultimately, we expect, the sale of cellular transceiver modules and associated wireless services.
On September 10, 2019, the Company changed its name from Appliance Recycling Centers of America, Inc. to JanOne Inc. and announced that it intended to broaden its business perspectives to include developing new and highly innovative solutions for ending the opioid epidemic. From digital technologies to educational advocacy to revolutionary painkilling drugs that address a multibillion dollar a year market, the Company intends to champion new initiatives to combat the opioid crisis, which claims tens of thousands of lives each year. The new name, JanOne, was strategically chosen to express the start of a “new day” in the fight against the opioid epidemic. January First is the first day of a New Year—a day of optimism, resolution, and hope. JanOne affirms the Company’s new strategic commitment to fresh thinking and innovative means to assist in ending the worst drug crisis in our nation’s history. The Company also adopted a new Nasdaq ticker symbol, NASDAQ: JAN, a new CUSIP number, 03814F403, and a new website address – www.janone.com. The information contained in or accessible from our website is not incorporated into this prospectus, and you should not consider it part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
On December 28, 2019, we had 208 employees, of which 199 were full-time employees.
We were incorporated in Minnesota in 1983, although, through our predecessors, we began operating our legacy recycling business in 1976. On March 12, 2018, we reincorporated in the State of Nevada. Our principal office is located at 325 E. Warm Springs Road, Suite 102, Las Vegas, Nevada 89119.
Biotechnology
Overview
We are a clinical-stage biopharmaceutical company focused on becoming the leader in identifying, acquiring, licensing, developing, partnering and commercializing novel, non-opioid and non-addictive therapies to address the large unmet medical need for the treatment of pain. Our initial product candidate, JAN101 (formerly known as TV1001SR) is a potential treatment for Periphery Artery Disease (“PAD”), a vascular disease that affects more than 60 million people worldwide. We are also researching the potential impact our compound JAN101 could have in patients with COVID-19 as many doctors around the world and our company believes COVID-19 is a respiratory disease that directly affects the vascular system. We expect to commence Phase 2b clinical trials for the treatment of PAD in early 2021. It is expected that the investigational new drug application (“IND”) for JAN101 as a COVID-19 vascular complication treatment will be submitted to the U.S. Food and Drug Administration (the “FDA”) in the coming weeks.
5
JAN101
Generally
JAN101, formerly known as TV1001SR and/or TV1001, our advanced product candidate, is a patented oral, sustained release pharmaceutical composition of sodium nitrite and targets poor blood flow to the extremities, such as those with vascular complications of diabetes or PAD and treats pain. A conclusion from a round of human studies found JAN101 sustained release sodium nitrite prevents the prevalent reports of headaches by patients treated with an immediate release formulation of sodium nitrite. In a previous study of patients with PAD, 40 mg BID treatment with immediate release sodium nitrite led to a statistically significant reduction in reported pain while a 80 mg BID treatment had the more pronounced effect on bioactivity and Flow Mediated Dilation, a measure of vascular function. However, a number of subjects on both treatment groups reported headaches and dizziness following treatment. Although this did not result in subjects discontinuing treatment, JAN101 was developed to overcome this side effect. JAN101 was tested in a bridging study of diabetic neuropathy subjects and during that bridging study, the subjects did not report headaches or dizziness. Subjects in this bridge study also reported less pain following treatment and improvements in bioactivity (quantitative sensory testing, a measure of nerve function) were similar to the PAD study, where the 80 mg dose group had the greatest improvement in Flow Mediated Dilation. The ability to alleviate pain with BID treatment of JAN101 offers promise for a new non-addictive, non-sedating treatment of chronic pain.
Clinical studies in humans JAN101 Attributes
|
|
• |
Well established safety profile |
|
|
• |
Excellent bioavailability |
|
|
• |
Lack of induced tolerance |
|
|
• |
Non-narcotic |
JAN1010 does not mask pain, but instead treats the cause of pain by improving tissue and vascular dysfunction.
Benefits of Sodium Nitrite on Vascular Health
In initial research studies, sodium nitrite effectively restored ischemic tissue blood flow and was effective in a wide range of pathologies involving alterations of angiogenesis - development of new blood vessels - including diabetes, wound healing and tissue necrosis. Beneficial effects included enhancing angiogenesis, endothelial cell proliferation, and arteriogenesis. There is also a strong association between reduced circulating nitrite levels and cardiovascular diseases in humans. We describe some of the associations and beneficial effects of sodium nitrite/nitrite below.
Plasma nitrite levels are negatively correlated to cardiovascular disease
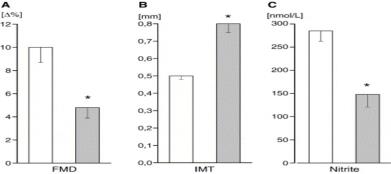

6
Plasma nitrite levels were inversely related to number of cardiovascular risk factors a subject had and decreased plasma nitrite was associated with decreased flow mediated vasodilation (FMD) and increased intimal medial thickness (IMT) (both indicators of vascular pathology). -Kleinbongard, et al. (2006) Free Radic Biol and Medicine 40:295-302
Plasma nitrite levels are reduced in diabetic and PAD patients
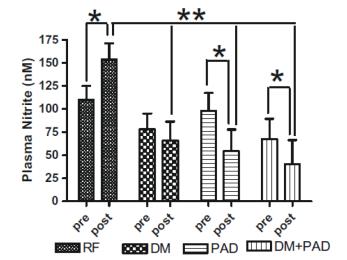
Exercise is a well-known stimulator of endothelial nitric oxide synthase activity, NO production that leads to increased plasma nitrite. In the study by Allen et al, these authors revealed that baseline plasma levels of nitrite were less in patients with diabetes mellitus (DM) or DM + PAD. Importantly, increases in plasma nitrite levels were not observed in either DM, PAD or DM + PAD patients after supervised exercise. These data reveal that baseline nitrite availability is compromised in DM patients and that supervised exercise is unable to increase plasma nitrite levels but actually results in a decrease in nitrite highlighting a physiological efficiency of this molecule. -Allen et al Nitric Oxide 2009 20:231-237
Skeletal Muscle Nitrite and Metabolite Levels are Reduced in Critical Limb Ischemia Patients
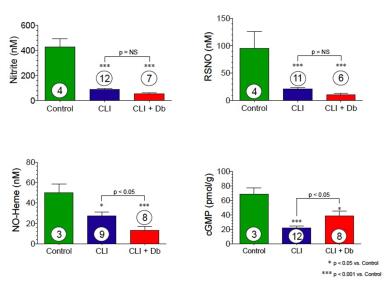
7
Skeletal muscle nitrite, nitrosothiol, nitric oxide-heme and cGMP are all significantly reduced in CLI patients. Diabetic patients with CLI show even further nitrite reductions.
In summary, nitrite levels in various cardiovascular and vascular diseases appear to be inversely related to the severity of the disease in humans:
|
|
• |
Lower nitrite levels are associated with higher level of heart failure; |
|
|
• |
Lower nitrite levels are observed in diabetic patients with PAD and are not compensated by exercise; and |
|
|
• |
Nitrite levels are lower in the muscles of patients with critical limb ischemia and are further reduced in diabetic subjects with critical limb ischemia. |
Given the association between low levels of circulating nitrite and human diseases, supplementation with sodium nitrite has been studied preclinically in animals. Below are summaries of some of the more important findings:
|
|
• |
Stimulates wound healing |
|
|
• |
Prevents tissue necrosis |
8
From Arya et al
Nitrite Therapy Selectively Increases Ischemic Tissue Vascular Density in a NO-dependent Manner
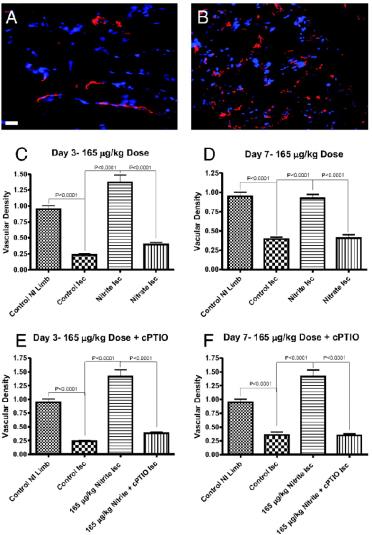
Chronic sodium nitrite therapy increases ischemic tissue vascular density in a NO-dependent manner. A and B show representative images of CD31 (red) and DAPI nuclear (blue) staining from sodium nitrite and sodium nitrate ischemic gastrocnemius muscle tissue at day 7. C and D report the vascular density of ischemic gastrocnemius muscle tissue at days 3 and 7 for 165 μg/kg sodium nitrite and nitrate treatments, respectively. E and F demonstrate the vascular density of ischemic gastrocnemius muscle tissue at days 3 and 7 from 165 μg/kg sodium nitrite plus carboxy PTIO. (Scale bar, 150 μm.) n = 10 mice per treatment group. Kumar D. et al. PNAS; 2008; 105:7540-7545.
9
Nitrite Therapy Augments Arterial Perfusion of Ischemic Tissue
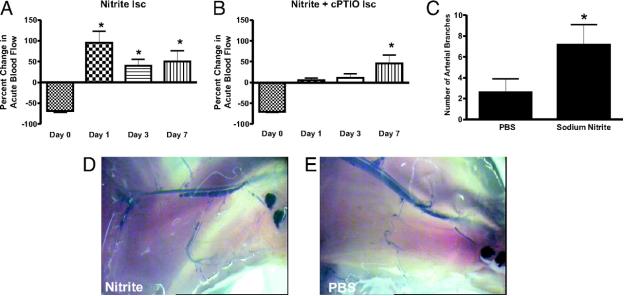
Chronic sodium nitrite therapy acutely increases ischemic tissue blood flow and stimulates arteriogenesis. A and B report 165 μg/kg sodium nitrite-induced acute changes in blood flow of chronically ischemic tissues at various time points with or without cPTIO, respectively. C reports the number of arterial branches between PBS and nitrite therapies. D and E illustrate vascular casting of the arterial vasculature in ischemic hind limbs of day 7 nitrite or PBS-treated mice, respectively. *, P < 0.01 vs. sodium nitrate. n = 10 mice per treatment group. Kumar D. et.al. PNAS;2008; 105:7540-7545
10
Nitrite Therapy Restores Diabetic Ischemic Hind-Limb Blood Flow and Promotes Wound Heal
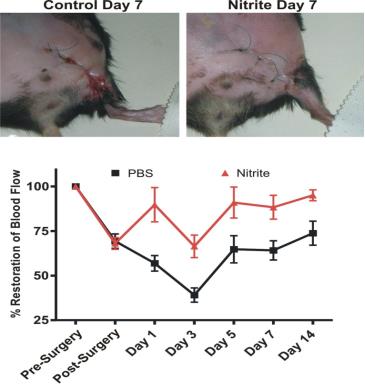
Unilateral femoral artery ligation was performed on 18-20 week old male Db/Db mice. Mice were randomized to PBS or sodium nitrite (165 μg/kg) therapy twice daily via I.P. injection. Laser doppler flowmetry was performed at the indicated time points. Increased wound dehiscence was noted in the PBS treated animals at day 7 but not in nitrite treated animals. (Bir et al Diabetes 2014, 63(1):270-81)
Nitrite Therapy Increases Diabetic Ischemia Induced Angiogenesis
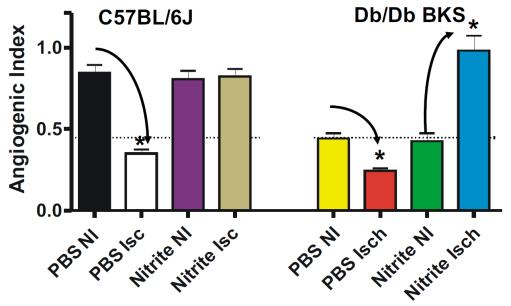
11
Nitrite therapy prevented ischemia mediated endothelial cell density loss in normal C57BL/6J ischemic limbs. Nitrite therapy significantly restored endothelial cell density in ischemic limbs of diabetic mice to normal C57BL/6J levels compared to PBS therapy of non-ischemic and ischemic conditions. These data suggest that nitrite therapy may be useful in attenuating microvascular rarefaction due to loss of nitric oxide that is observed during metabolic dysfunction (Frisbee JC AJP Integr Comp Physiol 2005 289(2):R307-16; Stepp et al Microcirculation 2007 14(4-5): 311-6)
Delayed Nitrite Therapy Restores Ischemic Hind-Limb Blood Flow
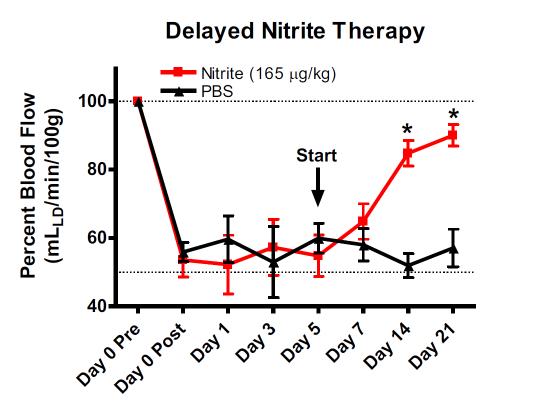
Studies were performed to determine whether nitrite mediated therapy would be effective in tissue that had been left ischemic for 5 days after femoral artery ligation. Femoral artery ligation was performed in C57BL/6J mice and the animals randomized to either PBS or sodium nitrite therapy 5 days after artery ligation. Treatments were given b.i.d. via I.P. injection. Ischemic limb blood flow was measured using laser doppler flowmetry. (Bir et al Diabetes 2014, 63(1):270-81)
12
Delayed nitrite therapy increases SPY angiogram arteriogenesis

Delayed nitrite therapy increases SPY angiogram arteriogenesis. Representative temporal SPY angiogram image stills (3–6s) are shown at 11 days following ligation and 6 days after beginning therapy (either
PBS or sodium nitrite). Left: PBS control angiogram. Right: sodium nitrite angiogram following injection of ICG. n = 5 animals per cohort. Circles identify limb anatomical regions of vascular blush, whereas arrows indicate perfused vessels that progressively occur over time.
Bir S C et al. Am J Physiol Heart Circ Physiol 2012;303:H178-H188
13
Nitrite Therapy Prevents Tissue Necrosis in Aged Db/Db Mice
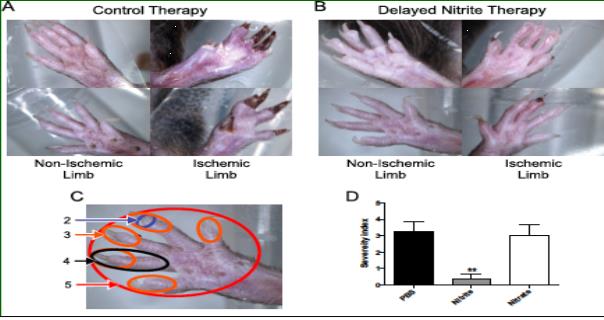
Delayed sodium nitrite (165 ug/kg) or control PBS therapy was stated 5 days post femoral artery ligation in 9 month old Db/Db mice. Nitrite therapy significantly prevented tissue necrosis (panel B) compared to control PBS therapy (panel A). Panel D reports tissue necrosis severity as a function of degree of limb and digit involvement. Nitrite therapy but not PBS control or sodium nitrate significantly prevented tissue necrosis. (Bir et al Diabetes 2014, 63(1):270-81)
Nitrite and Hind Limb Ischemia Summary
Sodium nitrite has long been known to be a potent vasodilator (transiently increasing blood vessel diameter) that can lead to a drop in blood pressure when given acutely. The above studies indicate that chronic administration at low doses, promotes angiogenesis, unlike single one-time nitrite therapy which does not stimulate angiogenesis. In addition, these studies and a large number of other studies not reviewed above, show:
|
|
• |
Nitrite therapy is very specific, acting only in damaged, ischemic tissue; |
|
|
• |
Delayed nitrite therapy effectively restores ischemic tissue blood flow; |
|
|
• |
Nitrite therapy is effective in a wide range of pathologies involving alterations of angiogenesis including critical limb ischemia, heart failure, and tissue necrosis; |
|
|
• |
Nitrite supplementation has had positive effects in various diabetes models, including diabetic nephropathy and diabetic wound healing; |
|
|
• |
Beneficial effects center on enhancing angiogenesis, endothelial cell proliferation, and arteriogenesis; and |
|
|
• |
Sustained release nitrite therapy, unlike immediate release therapy, does not lead to vasodilation or a drop in blood pressure. |
14
Our Product Candidate JAN101
Our product candidate is designed to treat diseases associated with poor vascular function. The following table summarizes our current product candidate pipeline:
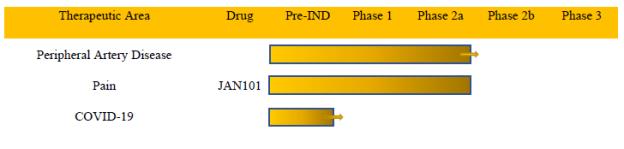
Therapeutic Area Peripheral Artery Disease Pain COVID-19 Drug JAN101 Pre-IND Phase 1 Phase 2a Phase 2b Phase 3
The Company intends to file an Investigational IND with the FDA for COVID-19 in the coming weeks and a protocol amendment to carry out a large Phase 2 trial in Peripheral Artery Disease patients early next year.
Pain
Pain is a protective reaction that alerts the body to the presence of actual or potential tissue damage so that necessary corrective responses can be mounted. The National Institutes of Health (the “NIH”) defines chronic pain as pain that persists beyond the normal healing time of an injury or that persists longer than three months. It is estimated that chronic pain affects 100 million individuals in the US and over 1.5 billion people worldwide, thus more people suffer from chronic pain than diabetes, heart disease and cancer combined (Cowen Therapeutic Categories Outlook March 2019). Chronic pain exacts a tremendous cost in terms of direct treatment and rehabilitation expenditures, lost worker productivity, prevalent addiction to opioid-based drugs, and emotional and financial burden for patients and their families. According to an Institute of Medicine of the National Academies report, pain is a significant public health problem in the United States that costs society between $560 and $635 billion annually. Despite the magnitude of the pain problem, innovation in the development of therapeutic solutions has been largely absent. Since 2010, there have been 20 approvals by the FDA for the treatment of pain, of which 12 were opioid variants, one was an extended release generic corticosteroid, five were variants of aspirin, and two were variants of other existing drugs. We are developing a novel product candidate designed to overcome the limitations of current treatment options for patients with PAD who suffer from chronic pain. According to a research study by Stanford University more than 24% of patients with PAD are at risk of high opioid use. By treating pain at the source and present patients and physicians with better and safer treatment alternatives we expect to minimize opioids at the prescription pad. Given the properties of JAN101, we have made the strategic decision to initially focus on pain associated with PAD by treating the underlying cause of PAD.
Peripheral artery disease
Peripheral artery disease is a general term for conditions in which arterial blood flow to the limbs are partially blocked. When there is less blood present in the extremities relative to demand, muscle pain and fatigue result, especially in the calf, which is also known as intermittent claudication. In many patients, pain and fatigue are relieved through rest. Roughly half of patients with PAD are asymptomatic. The most common cause of PAD / intermittent claudication is atherosclerosis. Diabetes, chronic kidney disease, hypertension, and smoking are all risk factors which can increase the likelihood of PAD. In atherosclerosis, fat deposits (plaques) build up along arterial walls, resulting in a reduction in blood flow in the legs. This same process can cause strokes if the arteries leading up to the brain are affected.
15
Because of the high rate of asymptomatic patients, prevalence figures vary widely. Some estimate that up to 200 million worldwide have PAD, ranging from asymptomatic disease to severe. Prevalence increases as a function of patient age, rising sharply after the age of 60. Thus, in countries with an aging population, it is expected that the prevalence of PAD will only increase. There is also a strong ethnic and racial component to PAD prevalence, which may be due to cultural differences in diet and exercise, along with genetic differences. Some suggest a prevalence of 8-12 million in the US alone, with roughly a third experiencing pain when walking, which improves upon resting. The diagnosis of PAD usually begins with patient complaints of pain in the extremities. If the patient is already being treated or monitored for diabetes or other risk factors, then the physician will check for a weak or absent pulse in the extremity. Decreased blood pressure, poor wound healing, and whooshing sounds in the legs (via stethoscope) are also tell-tale signs of PAD / intermittent claudication. Angiograms, electrocardiograms, and ultrasounds can also be used to image and confirm the diagnosis.
The non-drug treatment of PAD / intermittent claudication may be divided into four general categories:
|
|
• |
Lifestyle – Primarily changes in diet and smoking cessation. |
|
|
• |
Exercise – Patients who walk, cycle, stretch, or swim can experience marked improvement. Formal programs involving treadmills and track walking (usually 3-5 times per week) are frequently provided to patients. However, if the pain is triggered by exercise (claudication) and is significant, it can discourage the patient from exercise. |
|
|
• |
Angioplasty – A procedure by which the affected artery is stretched with a balloon-like device. This procedure has limited effectiveness and is reserved for severely blocked arteries. |
|
|
• |
Bypass Surgery – Arteries which are beyond angioplasty can be bypassed entirely. This procedure is typically reserved for cases where the blockage is considered very long (~10 centimeters) and nearly complete. |
The underlying condition, however, is not addressed by surgery. Surgical approaches will not, in the long run, improve exercise capacity and walking distance. Only exercise itself, coupled with lifestyle changes and drug approaches, has this benefit.
16
Prescription drugs for the treatment of the underlying PAD may be divided into multiple categories, depending on the underlying condition and severity:
|
|
• |
Cholesterol-Lowering Agents - Statins and bile acid sequestrants. |
|
|
• |
Antiplatelet Medica1ons – Aspirin and related drugs, such as clopidogrel. Cilostazol also has antiplatelet properties. |
|
|
• |
Antihypertensives – Patients with underlying high blood pressure can and will receive any number of medications to reduce blood pressure, such as ACE inhibitors and diuretics. |
|
|
• |
Diabetes Therapies – While a substantial portion of PAD patients may have pre-diabetes or fulminant diabetes, it is unknown of aggressive treatment of diabetes has a positive effect on PAD. |
|
|
• |
Pain – To our knowledge, no drugs are specifically indicated for PAD-associated pain. Pentoxifylline, for example, is indicated “…for the treatment of patients with intermittent claudication on the basis of chronic occlusive arterial disease of the limbs.” (Sanofi-Aventis U.S. LLC, 2010) However, the evidence supporting the effectiveness of pentoxifylline is mixed. Short-term courses of NSAIDs, such as ibuprofen may be used, provided the patient is not on another anticoagulant like aspirin. Non-drug pain relievers, such as TENS and massage, may also be used in these patients. Opioids may also be used which creates a risk for addiction and potential misuse at the medicine cabinet by family members. |
The lack of any truly effective treatment of PAD, along with encouraging early trial results using JAN101 on both improving vascular function and reducing pain in PAD patients, has created an opportunity to potentially treat this large unmet medical need. By improving vascular function, JAN101 has the potential to reduce associated pain and improve PAD patients’ quality of life.
17
COVID-19
Coronavirus disease (COVID-19) is an infectious disease caused by a newly discovered coronavirus.
Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Older people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. The COVID-19 virus spreads primarily through droplets of saliva or discharge from the nose when an infected person coughs or sneezes. At the time of filing this Form 10-K, there are no specific vaccines or treatments for COVID-19. However, there are many ongoing clinical trials evaluating potential treatments and vaccines.
One of the hallmarks of severe cases of COVID-19 is acute respiratory distress syndrome (“ARDS”), a rapid, widespread inflammation of the lungs that can lead to respiratory failure and death. In addition to the widely reported lung injuries associated with COVID-19, clinicians around the world are reporting that the disease also could be causing cardiac injuries in patients that sometimes lead to cardiac arrest. Kidney damage also is becoming a commonly reported issue among COVID-19 patients.
Alan Kliger, a nephrologist at the Yale School of Medicine, found early data showed 14% to 30% of ICU COVID-19 patients in New York and Wuhan, China, lost kidney function and later required dialysis. Similarly, a study published in the journal Kidney International found that nine of 26 people who died of COVID-19 in Wuhan had acute kidney injuries, and seven had units of the new coronavirus in their kidneys.
A study in May, 28 2020 in the New England Journal published research detailing the post-mortem features of seven patients who died of COVID-19 provides critical insights, including evidence of extensive damage to the lining of the blood vessels, abnormal blood vessel growth in the lungs and widespread blood clotting. The study led by Steven Mentzer, HMS professor of surgery at Brigham and Women’s Hospital, and done in collaboration with a team of international researchers tissue analysis showed that infection with SARS-CoV-2, the virus that causes COVID-19, caused severe damage to the endothelial cells that line blood vessels and triggered widespread blood clotting. The team also identified signs of a distinctive pattern of vascular disease progression in some cases of COVID-19 compared with patterns seen in equally severe influenza virus infection. The findings highlight these key takeaways:
|
|
• |
While caused by a respiratory virus, COVID-19 manifests as a vascular disease that leads to severe injuries to blood vessels throughout the lungs. The damage to vascular cells may help explain why serious blood clotting has been observed in many patients. |
|
|
• |
The substantial new blood vessel growth seen in the lungs of COVID-19 patients occurs primarily through a mechanism known as intussusceptive angiogenesis—the splitting of existing blood vessels to form new ones—perhaps as a repair response to blood clotting and blood vessel damage, according to the authors. |
Damaged blood vessels may also underlie other problems, such as COVID toe, multisystem inflammatory syndrome in children (MIS-C), stroke and other seemingly unrelated problems seen with COVID-19.
Our Team
Tony Giordano PhD, our Chief Scientific Officer, joined the company in December 2019. Dr. Giordano joined JanOne from the Cleveland Clinic, the No.2 rated hospital in the country, where he served as Senior Director of Special Projects in the Business Development group. Dr. Giordano has extensive experience in commercialization and drug development, having served as Vice President or President of seven different biotechnology companies he co-founded, including companies developing platform technologies, a cancer vaccine, and Alzheimer’s Disease and cardiovascular therapies. He has managed numerous clinical trials and the launch of a medical food product. Dr. Giordano has also served as an Associate Professor and Assistant Dean of Research and Business Development at LSU Health Sciences Center in Shreveport, where he led the licensing efforts at the campus and at Abbott Labs, where in addition to serving as a Senior Research Scientist, he was involved in technology assessment activities. Dr. Giordano has a PhD focused in Molecular Genetics from Ohio State University and completed Fellowships at the NCI and NIA.
18
Dr. Amol Soin, our Chief Medical Officer, joined the Company in January 2020. Dr. Soin is considered one of the nation's top pain experts and is the Founder and Chairman of the Ohio Pain Clinic. Dr. Soin brings significant expertise for treating neuropathic and chronic pain and extensive research experience for non-opioid, nonaddictive pain solutions to the JanOne management team. In his role as Chief Medical Officer, Dr. Soin will guide JanOne's drug development activities, manage clinical research, set patient safety standards, and ensure regulatory compliance. In addition, Dr. Soin will play an integral role in establishing partnerships and drug candidate selection as the company expands its pipeline. Dr. Soin received his undergraduate degree from University of Akron, his MBA from University of Tennessee, his MD from Northeastern Ohio Universities College of Medicine, his master's in science from Brown University and also has studied at Dartmouth College. He is board certified in anesthesiology and pain medicine and a fellow of interventional pain management at the World Institute of Pain, and he served as a pain management fellow at the Cleveland Clinic, the oldest and largest academic pain management department in the United States. The founder and chairman of the Ohio Pain Clinic, Dr. Soin has also held several prestigious positions including President of the Ohio Society of Interventional Pain Physicians, president of the American Society of Interventional Pain Physicians Foundation, President of the Society of Interventional Pain Management Surgery Centers and president – elect of TriState Pain Society. He was appointed by Governor Kasich to the Ohio Medical Board in 2012 to two 5 year terms and has served as the Ohio Medical Board's president where he was instrumental in passing statewide rules and guidelines to help the opioid crisis.
In November 2019, we formed a Scientific Board of Advisors (the “SBA”) and the following doctors and scientist currently sit on the SBA:
Chris Kevil, Ph.D., Chair of the Scientific Advisory Board -- Dr. Kevil, an internationally known expert in vascular pathophysiology, PAD, and nitric oxide biology, discovered the role of sodium nitrite in promoting angiogenesis that led to the development of TV1001 now known as Jan101. Dr. Kevil earned his Ph.D. degree from LSU Health Shreveport in Molecular and Cellular Physiology followed by a fellowship at the University of Alabama at Birmingham (UAB) with an emphasis on redox pathophysiology. Returning to LSU Health Shreveport in the Department of Pathology, he established cutting edge research programs regarding redox biology regulation of peripheral vascular diseases. This led to ground-breaking insights on how glutathione, nitrite/nitric oxide, and hydrogen sulfide regulate vascular health during ischemia.
Edgar Ross, MD -- Dr. Ross is the current Director of the Pain Management Center at Brigham and Women's Hospital and a professor of anesthesia at Harvard Medical School. Dr. Ross is recognized as Castle Connolly's America's top doctors for the fifth year in a row. In addition to serving as chairman of Pfizer's partnership on pain, Dr. Ross also has served as a member of the Blue Cross and Blue Shield Opioid Prescribing Policy Committee.
Rakesh Patel, Ph.D. -- Dr. Patel is currently Vice Chair for Research, Department of Pathology, and Director of the Center for Free Radical Biology at the University of Alabama at Birmingham (UAB). Most noted is his research to understand the molecular basis of nitric oxide, and nitrite interactions with organs and red blood cells. Patel is also known for his work to understand the impacts on the biological process associated with blood flow regulation and pulmonary function.
Timothy Ness, MD, Ph.D. -- Dr. Ness is Professor Emeritus and former Pain Treatment Division Chief, Director of Pain Research and Vice Chair for Clinical Research in the Department of Anesthesiology and Perioperative Medicine at the University of Alabama at Birmingham (UAB) He has served as a clinical research expert on pain for the National Institutes of Health (NIH), Food and Drug Administration (FDA) advisory panels, the Veterans Administration (VA), and various international research institutes. He has served on the American Pain Society and the American Society of Regional Anesthesia and Pain Medicine Board of Directors. He is currently funded by the NIH.
Alan Kaye, MD, PhD, DABA, DABPM, DABIPP -- Dr Kaye is the Professor and Chairman of the Department of Anesthesiology at LSU Health Sciences Center in New Orleans since January 2005. Before LSU, he was Professor and Chairman of the Texas Tech University Health Sciences Center Department of Anesthesiology in Lubbock, Texas. Prior, he was the Medical Director of the Greater New Orleans Surgical Center, the Director of Resident Recruitment, Acting Program Director and an Attending Staff of the Department of Anesthesiology at Tulane University Medical Center in New Orleans. He received two BS degrees and a MD degree from the University of Arizona. He also completed a pain management fellowship at Texas Tech Health Sciences Center. He is Board
19
Certified as a Consultant in Anesthesiology and has a special certificate in Pain Management for the American Board of Anesthesiology. He is also a Diplomate of the American Board of Pain Medicine and the American Board of Interventional Pain Physicians. Dr. Kaye completed his PhD in pharmacology in May 1997. His thesis title was "Pharmacology of Angiotensin Peptides and Nonpeptide Agonists in the Pulmonary Vascular Bed of the Cat and of the Rat." He was awarded first place in the National Student Research Forum as a resident and has authored or co-authored over 150 abstracts and 200 manuscripts and book chapters in the fields of pulmonary vascular pharmacology and anesthesiology. He has had a lifelong interest in education and teaching medical students and residents. He serves on a number of national committees including as a National Board of Directors of ASIPP and ABIPP. He is editor-in-chief of the journal Pain Physicians and is on the FDA Advisory Board on Anesthetics and Analgesics. He was an Associate National Board Examiner in Anesthesiology
John Cooke, MD, Ph.D. -- is the Chair of the Department of Cardiovascular Sciences at the Houston Methodist Research Institute, Director of the Center for Cardiovascular Regeneration, and Medical Director of the RNA Therapeutics Program in the Houston Methodist DeBakey Heart and Vascular Center in Houston, Texas. He trained in cardiovascular medicine and obtained a Ph.D. in physiology at the Mayo Clinic. He was recruited to Harvard Medical School as an assistant professor of medicine. In 1990, he was recruited to Stanford University to spearhead the program in vascular biology and medicine, and was appointed professor in the Division of Cardiovascular Medicine at Stanford University School of Medicine, and associate director of the Stanford Cardiovascular Institute until his recruitment to Houston Methodist in 2013. Dr. Cooke has published over 500 research papers, position papers, reviews, book chapters and patents in the arena of vascular medicine and biology with over 30,000 citations. He has served on national and international committees that deal with cardiovascular diseases, including the American Heart Association, American College of Cardiology, Society for Vascular Medicine, and the National Heart, Lung and Blood Institute. He has served as president of the Society for Vascular Medicine, as a director of the American Board of Vascular Medicine, and as an associate editor of Vascular Medicine.
Our Strategy
Our mission is to develop and commercialize novel, non-opioid, and non-addictive therapies to safely and effectively address the significant unmet medical need of chronic pain or treat conditions that cause pain. The principal elements of our strategy to achieve this mission are the following:
|
|
• |
License, acquire, develop, and create novel, non-opioid and non-addictive therapies by leveraging our understanding of pain biology to address the large and growing problem of pain. While innovation in medical sciences has led to exciting new treatment options in many disease areas, pain has seen limited innovation in recent years. We have a deep understanding of the pathophysiology of pain and diseases that cause pain. We intend to leverage this understanding to bring innovation in the pain treatment paradigm through targeted acquisitions of companies or assets in development. Our advisors and doctors have years of collective experience in leadership positions at institutions and substantial scientific experience, and understand the complexity of designing and executing clinical trials for and developing therapies. |
|
|
• |
Advance the development of our lead product candidate, JAN101, designed for the treatment of patients with PAD and pain associated with the disease. There are limited therapeutic options available for patients with PAD and we believe that JAN101 has the potential to transform the standard of care to a twice a day pill to substantially improve moderate to severe PAD. The company plans to engage a contract research organization (“CRO”) in early 2021 and begin enrolling subjects for the first Phase 2b trials for JAN101, and we expect to report topline results promptly following receipt of the data from the CRO. |
|
|
• |
Leverage clinical activity of JAN101 to expand into new indications, including complications associated with COVID-19. We believe that JAN101 may have utility in treating vascular complications in patients with COVID-19 as we believe COVID-19 is an endothelial cell disease which manifests its complications in the vascular system and major organ causing complications in recovered patients. In November 2020, we filed an IND for our COVID-19 indication (which was subsequently converted to a pre-IND) and begin our study if and when we receive approval from the FDA. We plan to release more information regarding our COVID-19 study once the FDA has cleared the IND. |
20
|
|
• |
Advance our product candidates through clinical development and pursue development of additional product candidates through acquisitions. Our objective is to build a well-balanced, multi-asset portfolio targeting the large population of patients with chronic and acute pain. To achieve this, in addition to JAN101, we intend to pursue partnerships, licensing agreements, and potential acquisitions of other pharma companies. We continue our search for assets with indications where we believe they could have meaningful impact and address the large unmet medical need. In addition, we may choose to selectively in-license or acquire complementary product candidates by leveraging the insights, network, and experience of our team. |
|
|
• |
Maximize the commercial potential of all our product candidates. We currently intend to retain all commercial rights to JAN101 in the United States and selectively partner outside of the United States. Because we believe that PAD is an attractive market for many major pharmaceutical companies, we may sub-license or partner certain indications if we believe it may enhance stockholder value. As we continue to build and develop our product portfolio, we may opportunistically pursue strategic partnerships that maximize the value of our pipeline while seeking to develop other indications. |
|
|
• |
Leverage our management team background and expertise. We have assembled a team with extensive experience described above. |
Chronic Pain
The NIH defines chronic pain as pain that persists either beyond the normal healing time of an injury or longer than three months. We believe that chronic pain represents a significant public health crisis. In the United States, chronic pain affects approximately 40 million adults annually, which is greater than the annual prevalence of each of heart disease, cancer and diabetes. It is also estimated that pain leads to between $560 and $635 billion in healthcare and lost productivity costs each year. Chronic pain is the leading cause of long-term disability in the United States, and approximately 23 million adults in the United States experience severe pain over a three-month period. Globally the prevalence of chronic pain is even larger, with over 1 billion people worldwide affected each year. Common types of chronic pain include those of neuropathic and inflammatory origin and may involve the skin, muscles, joints, bones, tendons, ligaments, and other soft tissues. Chronic pain is associated with a variety of clinical conditions including, but not limited to, arthritis, spinal conditions, cancer, fibromyalgia, diabetes, surgical recovery, visceral injury and general trauma.
Pain is a necessary protective reaction that alerts the body to the presence of actual or potential tissue damage so that necessary corrective responses can be mounted. Pain is signaled by specialized cells in the peripheral nervous system called nociceptors, or pain-sensing fibers. These pain-sensing fibers normally transmit information about stimuli that approach or exceed harmful intensity from different locations in the body to the brain, which registers this information as a sensation of pain. In the case of tissue injury due to trauma or infection, pain accompanies the associated inflammation, persists for the duration of the inflammatory response, and aids healing by inhibiting use of the affected body part.
Pain also can modify the central nervous system such that the brain becomes sensitized and registers more pain with less provocation. This is called central sensitization. When central sensitization occurs, the nervous system goes through a process called wind-up and gets regulated in a persistent state of high reactivity. This persistent, or up-regulated, state of reactivity lowers the threshold for what triggers the sensation of pain and can result in the sensation of pain even after the initial injury might have healed.
When there is dysfunction in pain signaling, injury to the nervous system, or an unhealed injury, pain becomes no longer just a symptom, but a disease in itself.
Current Therapeutic Approaches to Treating Chronic Pain and Their Limitations
NSAIDs
Some of the most widely used therapies to treat chronic inflammatory pain are non-steroidal anti-inflammatory drugs, or NSAIDs. NSAIDs can have significant side effects that include gastrointestinal bleeding, gastritis, high blood pressure, fluid retention, kidney problems, heart problems and rashes. On April 7, 2005, the FDA announced a decision to require boxed warnings of potential cardiovascular risk for all NSAIDs.
21
Corticosteroids
Corticosteroids, or steroids, also possess anti-inflammatory properties and are commonly used in the practice of pain management, either systemically or locally, depending on the condition. Steroids work by decreasing inflammation and reducing the activity of the immune system. While steroids are commonly used, they may have numerous and serious side effects. These side effects may include allergic or hypersensitivity reactions, increased risk for infection, adrenal insufficiency, diabetes or decreased glucose tolerance, hypertension, loss of bone density, and loss of joint cartilage volume. In addition, steroids should not be administered when there is an infection present because steroids can inhibit the body’s natural infection-fighting immune response. Also, if a joint is already damaged or is subject to chronic deterioration, IA steroid injections are not likely to provide any long-term restorative benefit. For the above reasons, IA steroid injections are generally recommended to be administered no more often than every six weeks and not more than three to four times per year.
Opioids
Opioids are some of the most widely prescribed therapeutics for chronic and acute pain, and sales of these drugs have quadrupled between 1999 and 2010. According to a National Survey on Drug Use and Health report, in 2016 more than one third of adult Americans were prescribed opioids and 230 million opioid prescriptions were written that year in the United States. Opioids act by binding to specific receptors located on neurons in both the central and peripheral nervous system throughout the body including in the brain, spinal cord and other nervous tissue. Although they can be effective in providing pain relief, the increased medical use of opioids has been accompanied by an increase in the abuse and misuse of prescription opioids. In addition, for most patients, chronic opioid use is a poor option due to an intolerance to the many side effects, including nausea, vomiting, drowsiness and constipation, and the propensity for opioids to become less effective with long-term use. According to the Centers for Disease Control and Prevention, or CDC, almost two million individuals abused or were dependent on prescription opioids in 2014. CDC figures show that the number of opioid-related overdose deaths has quadrupled between 1999 and 2010, and currently approximately 40% of opioid overdose deaths in the United States involve a prescription opioid. This increase in prescription opioid-related deaths in the United States prompted President Trump to declare the opioid crisis a national Public Health Emergency in October 2017. Opioid abuse has become an epidemic in the United States, ranking as the nation’s second most prevalent illegal drug problem. These major issues create the need to find new approaches to treating chronic pain.
Our Approach to Treating PAD and Chronic Pain
The unmet medical need for treating PAD and chronic pain reflects the historic failure to develop novel classes of analgesics with comparable or greater efficacy, an acceptable level of adverse effects and a lower abuse liability than those currently available. Some of the reasons for this include the heterogeneity of chronic pain and its related conditions, and the complexity and diversity of the underlying pathophysiological mechanisms for pain. However, recent advances in the understanding of the neurobiology of pain are beginning to offer opportunities to identify new drug targets and develop new therapeutic strategies.
We have taken an innovative and targeted approach to identifying treatments for chronic pain that leverages our understanding of the pathophysiology of pain. Pain is variable—for example, it can be inflammatory or neuropathic in nature, and it may be localized to a specific area of the body or it may be generalized throughout. We believe that the most effective way to treat chronic pain is through therapies that specifically target the origin of the pain signal. We strive to maximize each of our product candidate’s potential based on its unique mechanism of action related to the origin of the pain signal.
A Randomized, Double-Blind Study of the Effects of a Sustained Release Formulation of Sodium Nitrite (SR-nitrite) on Patients with Diabetic Neuropathy
Background: Background: Sodium nitrite has been reported to be effective in reducing chronic peripheral pain.
Objectives: To evaluate the safety and efficacy of 40 and 80 mg, BID, of an oral sustained release formulation of sodium nitrite (SR-nitrite) in patients suffering from diabetic neuropathy, and to determine whether SR-nitrite would reduce the frequency of headaches reported previously by subjects receiving the same doses of an immediate release formulation. Study Design: Phase II, single-center, randomized, double-blind, placebo controlled clinical trial. Setting: The Ohio Pain Clinic and Kettering Medical Center.
22
Methods: Twenty-four patients were randomized to 40 mg or 80 mg SR-nitrite or placebo twice daily for 12 weeks. The primary objective was to determine whether headaches would be reduced using SR-nitrite. The primary efficacy endpoint was the mean difference in the change of the Neuropathic Pain Symptom Inventory (NPSI) pain score from baseline to that reported after 12 weeks of treatment. Secondary endpoints included changes from baseline for the Brief Pain Inventory (BPI) Scale, the RAND 36 questionnaire, Short Form McGill Questionnaire, daily patient reported score for neuropathic pain, changes in HbA1c, PulseOx and quantitative sensory testing. Results: The number of subjects reporting adverse events and the number of adverse events did not change with dose. There were no reports of treatment-related headaches. Although no significant differences were identified in patient responses to the questionnaires, a trend was observed. In the NPSI assessment, patients in the 40 mg and 80 mg dose group reported a 12.7% and 22.0% reduction in pain, respectively, compared to an 8.4% reduction by patients in the placebo group. A trend was also observed with the BPI total severity score. However, the 40 mg dosing group reported the greatest reduction in pain using the McGill Pain index and via patient logs of daily pain scores, where the mean of pain scores reported by subjects in the 40 mg group dropped by day 41 and generally stayed lower than the mean of scores reported by subjects in either of the other two groups. Patients in the 80 mg SR-nitrite group had an improvement in both Nerve Sensory Conductance and Nerve Sensory Velocity. No changes were observed in HbA1c levels or PulseOx.
Limitations: Small sample size.
Conclusion: Sustained release sodium nitrite prevents the prevalent reports of headaches by patients treated with an immediate release formulation of sodium nitrite. In a previous study of patients with peripheral arterial disease (PAD), 40 mg BID treatment led to a statistically significant reduction in reported pain, similar trends were observed at the end of the trial period for most of the pain questionnaires used in the study. The 80 mg BID treatment had the more pronounced affect on bioactivity (quantitative sensory testing), which was similar to the PAD study, where this dose group had the greatest improvement in FMD {AU: spell out FMD}. The ability to alleviate pain with BID treatment of SR-nitrite offers promise for a new non-addictive, non-sedating treatment of chronic pain and warrants further study.
Microcirculatory injury, which is common in diabetic patients, can lead to a number of problems. Prominent among these is diabetic peripheral neuropathy (DPN) (1,2). About 10% of patients will have evidence of DPN at the time they are initially evaluated, and almost 50% of diabetic patients will ultimately develop DPN. Of diabetic patients with DPN, 40% to 50% suffer from chronic pain as well as paresthesias, sensory loss, and weakness, and have at least an 8-fold increased risk of undergoing a distal lower extremity amputation compared to similar non-diabetics. Endothelial cells play an important part in the regulation of microcirculation, as they maintain vascular tone by secreting both vasodilators and vasoconstrictors. A central feature of diabetic microvascular disease (MVD) is endothelial dysfunction, which, in turn, plays an important role in the development and progression of DPN. The pathophysiological factors leading to endothelial dysfunction in diabetes include chronic hyperglycemia and protein glycolation, insulin resistance, inflammation, and increased oxidative stress. Studies have now shown a close relationship between endothelial dysfunction and diminished nitric oxide (NO) bioavailability. Endogenously produced NO has a half life measured in seconds, and is rapidly oxidized to nitrite (NO2–) and nitrate (NO3––) end products, the latter of which is biologically inert. In the presence of microcirculatory ischemia and endothelial cell dysfunction, however, endogenous NO production by eNOS is much more limited. In such circumstances, circulating NO2– can be non-enzymatically reduced to increase NO availability. In addition to serving as a circulating NO reservoir, nitrite itself has also been shown to have direct and potent vasodilatory effects in vitro and in vivo. The findings that NO2– mediates vasodilatation, both directly and through NO generation, has led to growing interest in the potential effectiveness of nitrite as a therapeutic agent in conditions associated with DPN and endothelial dysfunction. Such conditions include diabetic microvascular disease, DPN, and retinopathy, in which low levels of NO and NO2–, as well as elevated levels of nitrate (NO3), suggest that the complete oxidation of NO occurs during diabetes with insufficient NO2– reserves to restore NO bioavailability. Previous human studies with an oral formulation of NaNO2 have shown that administration twice daily improves vascular function. In the peripheral arterial disease study, subjects who received the lower dose of NaNO2 reported a significant reduction in pain. Although side effects were minimal, headaches and dizziness were reported by a large number of subjects, likely due to the rapid release of NaNO2 leading to vasodilation. An oral sustained-release formulation of NaNO2 (SR-nitrite) was developed in an attempt to overcome these problems and was tested in a porcine model of metabolic syndrome with critical limb ischemia. SR-nitrite-treated animals showed increased myocardial NO
23
bioavailability, diminished oxidative stress, and cytoprotection in ischemic tissue. Importantly, 24-telometry recordings of blood pressure showed no evidence of vasodilation. In the present study, we hypothesized that the SRnitrite would reduce or eliminate headaches reported in patients following administration of the immediate release formulation. Given the promising results on reducing pain in diabetic patients with peripheral arterial disease reported in the previous study, patients with diabetic neuropathy were utilized in this study to determine whether any trends in reducing pain could be observed. The study design was a randomized, placebo controlled, double-blind phase II study was carried out to investigate the safety and potential biological activity of multiple doses of an oral, sustained-release formulation of sodium nitrite (SR-nitrite; Theravasc Inc., Cleveland, OH, USA), BID in doses of 40 mg and 80 mg over a 12-week treatment period, in human subjects with diabetes and neuropathic pain in the lower extremities and feet. The trial was approved by the Copernicus IRB and listed on ClinicalTrials.gov: www.clinicaltrials.gov/ct2/show/NCT02412852. The study was funded by Theravasc Inc.
JAN101—Regulatory Strategy
Sodium Nitrite has been previously approved as one of the active components of cyanide poisoning antidote. This means the approval path for JAN101 is through a 505(b)(2) NDA, which we intend to pursue.
JAN101—Commercial Strategy
We currently intend to use third party providers and manufacturers in the United States to effectively support the commercialization JAN101, if we are successful in obtaining FDA approval. We believe that we can cost effectively promote JAN101 to the patients suffering from PAD. We anticipate our commercial operation to include outside sales management, outside sales support, distribution support and an internal marketing group. Additional requisite capabilities will include focused management of key accounts, such as managed care organizations, group purchasing organizations, and government accounts. We intend to selectively partner with third parties with vast experience in the space as we have been partnering for every aspect of development.
Competition
The biotechnology and pharmaceutical industries are characterized by extensive research and development efforts, rapidly advancing technologies, intense competition, and a strong emphasis on proprietary products. We are currently focused on the development and commercialization of our asset pipeline of novel, non-opioid and non-addictive therapies for PAD. The number of patients suffering from chronic PAD is large and growing. While we believe that our product candidate and our Chief Scientific Officers development experience and scientific knowledge provide us with competitive advantages, we face potential competition from many different sources, including pharmaceutical, biotechnology, and specialty pharmaceutical companies either marketing or developing therapeutics to treat chronic pain. Academic research institutions, governmental agencies, as well as public and private institutions are also potential sources of competitive products and technologies. Our competitors may have significantly greater financial resources, robust drug pipelines, established presence in the market and expertise in research and development, manufacturing, pre-clinical and clinical testing, obtaining regulatory approvals and reimbursement and marketing approved products than we do. These competitors also compete with us in recruiting and retaining qualified clinical, regulatory, scientific, sales, marketing and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. The key competitive factors affecting the success of all of our product candidates, if approved, are likely to be their efficacy, durability, safety, price and the availability of reimbursement from government and other third-party payors.
Significant competition exists in the PAD pain field. Although we believe our approach to developing novel treatments for pain is unique from most other existing or investigational therapies, such as non-steroidal anti-inflammatory drugs (“NSAIDs”), corticosteroids and opioids, we will need to compete with all currently available and future therapies within the indications where our development is focused. With respect to JAN101, the main classes of marketed products that are available for the treatment of PAD pain include NSAIDs and opioids. Furthermore, numerous monoclonal antibodies targeting nerve growth factor, or NGF inhibitors, are in clinical development, including two product candidates in Phase 3.
There are a number of companies developing or marketing therapies for the treatment and management of pain that may compete with our current product candidate, including many major pharmaceutical and biotechnology companies. Among the companies that currently market or are developing therapies that, if approved, our product
24
candidates would potentially compete with include: Acorda Therapeutics, Assertio Therapeutics, Biogen, Cara Therapeutics, Eli Lilly and Company, Endo Pharmaceuticals, Flexion Therapeutics, Grunenthal, Horizon Pharma, Janssen Research & Development, Merck & Co., Novartis, Pacira Pharmaceuticals, Pain Therapeutics, Pfizer, Purdue Pharma, Sanofi, Trevena and Vertex Pharmaceuticals.
Intellectual Property
Our success depends in large part upon our ability to obtain and maintain proprietary protection for our products and technologies, and to operate without infringing or otherwise violating the proprietary rights of others. We endeavor to protect our products using a combination of intellectual property protections and available government regulatory and marketing exclusivities afforded to new medicines. For example, we endeavor to protect our products by, among other methods, filing U.S., and potentially in the future, foreign, patent applications related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also use other forms of protection, such as confidential information, trade secrets and know-how, and trademarks to protect our intellectual property, particularly where we do not believe patent protection is appropriate or obtainable.
The proprietary nature of, and protection for, our product candidates, processes and know-how are important to our business. Our policy is to pursue, maintain and defend intellectual property rights, and to protect the technology, inventions, and improvements that are commercially important to our business.
Trade Secrets and Other Proprietary Information
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. For example, we have developed methods for the more efficient manufacture of sustained released sodium nitrite tablets. We seek to protect our proprietary information, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and commercial partners.
LSU License Agreement
On November 19, 2019, we entered into a Patent and Know How License Agreement (the “License Agreement”) with UAB Research Foundation (“UABRF”), TheraVasc, Inc. (“TheraVasc”), and the Board of Supervisors of Louisiana State University and Agricultural and Mechanical College, acting on behalf of LSU Health Sciences Center at Shreveport (“LSU Health Shreveport”, together with UABRF and TheraVasc, the “Licensors”). Under the License Agreement, the Licensors have agreed to grant to JanOne an exclusive, worldwide license, including the right to sublicense, to the Licensors’ patent rights and know-how related to the Licensors’ sustained release formulation of sodium nitrite. Under the License Agreement, have agreed to pay a non-refundable upfront license fee and certain milestone payments upon the achievement of certain milestones of up to approximately $6.5 million and certain royalty payments and annual license maintenance fees. The License Agreement requires us to use commercially reasonable efforts to develop and commercialize JAN101.
Commercial Operations
We currently have no marketing and sales organization. We have retained global rights to our product candidate, and, if one of our product candidates is approved by the FDA, expect to access the market in the United States we expect that our sales force will be supported by sales management, internal sales support, an outside marketing group and distribution support. We intend to invest in our commercial capabilities prudently by focusing our marketing efforts on the physician subspecialties that treat patients with PAD. These physicians include, but are not limited to, pain management specialists, rheumatologist, surgeons and sports medicine physicians. We will also evaluate licensing and partnering with third parties to help us reach other sales channels and geographic markets inside and outside of the United States.
25
Government Regulation
The FDA and comparable regulatory authorities in state and local jurisdictions and in other countries impose substantial and burdensome requirements upon companies involved in the clinical development, manufacture, marketing and distribution of drugs, such as those we are developing. These agencies, and other federal, state and local entities regulate, among other things, the research and development, testing, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion, distribution, post-approval monitoring and reporting, sampling and export and import of our product candidates.
U.S. Government Regulation of Drug Products
In the United States, the FDA regulates drugs under the FDCA and its implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
|
|
• |
completion of pre-clinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practice, or GLP, regulations; |
|
|
• |
submission to the FDA of an IND which must become effective before human clinical trials may begin; |
|
|
• |
approval by an IRB at each clinical site before each trial may be initiated; |
|
|
• |
performance of adequate and well-controlled human clinical trials in accordance with GCP requirements to establish the safety and efficacy of the proposed drug product for each indication; |
|
|
• |
submission to the FDA of an NDA; |
|
|
• |
satisfactory completion of an FDA advisory committee review, if applicable; |
|
|
• |
satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP requirements and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; |
|
|
• |
satisfactory completion of FDA audits of clinical trial sites to assure compliance with GCPs and the integrity of the clinical data; |
|
|
• |
payment of user fees and securing FDA and approval of the NDA; and |
|
|
• |
compliance with any post-approval requirements, including the potential requirement to implement a REMS and the potential requirement to conduct post-approval studies. |
26
Pre-clinical Studies
Pre-clinical studies include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess potential safety and efficacy. An IND sponsor must submit the results of the pre-clinical tests, together with manufacturing information, analytical data and any available clinical data or literature, among other things, to the FDA as part of an IND. Some pre-clinical testing may continue even after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence. Clinical holds also may be imposed by the FDA at any time before or during clinical trials, due to safety concerns about on-going or proposed clinical trials, or non-compliance with specific FDA requirements, and the trials may not begin or continue until the FDA notifies the sponsor that the hold has been lifted.
Clinical Trials
Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution. Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health for public dissemination on their www.clinicaltrials.gov website. The information contained in, or accessible through, this website does not constitute a part of this prospectus. We have included this website address in this prospectus solely as an inactive textual reference.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
|
|
• |
Phase 1: The drug is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness. |
|
|
• |
Phase 2: The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
|
|
• |
Phase 3: The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product. |
Post-approval trials, sometimes referred to as Phase 4 trials, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA.
The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. In addition, some clinical trials are overseen by an independent group of qualified experts organized by the sponsor, known as a data safety monitoring board or committee. Depending on its charter, this group may determine whether a trial may move forward at designated check points based on access to certain data from the trial.
27
During the development of a new drug, sponsors are given opportunities to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase 2, and before an NDA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and the FDA to reach agreement on the next phase of development. Sponsors typically use the meetings at the end of the Phase 2 trial to discuss Phase 2 clinical results and present plans for the pivotal Phase 3 clinical trials that they believe will support approval of the new drug.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. In addition, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
While the IND is active and before approval, progress reports summarizing the results of the clinical trials and non-clinical studies performed since the last progress report must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and investigators for serious and unexpected suspected adverse events, findings from other studies suggesting a significant risk to humans exposed to the same or similar drugs, findings from animal or in vitro testing suggesting a significant risk to humans, and any clinically important increased incidence of a serious suspected adverse reaction compared to that listed in the protocol or investigator brochure.
United States Review and Approval Process
The results of product development, pre-clinical and other non-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The submission of an NDA is subject to the payment of substantial user fees; a waiver of such fees may be obtained under certain limited circumstances. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. Under the Prescription Drug User Fee Act, or PDUFA, guidelines that are currently in effect, the FDA has a goal of ten months from the date of “filing” of a standard NDA for a new molecular entity to review and act on the submission. This review typically takes twelve months from the date the NDA is submitted to FDA because the FDA has approximately two months to make a “filing” decision after it the application is submitted. The FDA conducts a preliminary review of all NDAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
The FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect one or more clinical trial sites to assure compliance with GCP requirements.
After the FDA evaluates an NDA, it will issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with prescribing information for specific indications. A Complete
28
Response Letter indicates that the review cycle of the application is complete and the application will not be approved in its present form. A Complete Response Letter usually describes the specific deficiencies in the NDA identified by the FDA and may require additional clinical data, such as an additional pivotal Phase 3 trial or other significant and time-consuming requirements related to clinical trials, non-clinical studies or manufacturing. If a Complete Response Letter is issued, the sponsor must resubmit the NDA or, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information are submitted, the FDA may decide that the NDA does not satisfy the criteria for approval.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. In addition, the FDA may require a sponsor to conduct Phase 4 testing, which involves clinical trials designed to further assess a drug’s safety and effectiveness after NDA approval, and may require testing and surveillance programs to monitor the safety of approved products which have been commercialized. The FDA may also place other conditions on approval including the requirement for REMS, to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA must submit a proposed REMS. The FDA will not approve the NDA without an approved REMS, if required. A REMS could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Marketing approval may be withdrawn for non-compliance with regulatory requirements or if problems occur following initial marketing.
The FDASIA, made permanent the Pediatric Research Equity Act, or PREA, which requires a sponsor to conduct pediatric clinical trials for most drugs, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs and supplements must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must evaluate the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or FDA may request a deferral of pediatric clinical trials for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the drug is ready for approval for use in adults before pediatric clinical trials are complete or that additional safety or effectiveness data needs to be collected before the pediatric clinical trials begin. The FDA must send a non-compliance letter to any sponsor that fails to submit the required assessment, keep a deferral current or fails to submit a request for approval of a pediatric formulation.
Special FDA Expedited Review and Approval Programs
The FDA has various programs, including Fast Track Designation, accelerated approval, priority review, and breakthrough therapy designation, which are intended to expedite or simplify the process for the development and FDA review of drugs that are intended for the treatment of serious or life threatening diseases or conditions and demonstrate the potential to address unmet medical needs. The purpose of these programs is to provide important new drugs to patients earlier than under standard FDA review procedures.
To be eligible for a Fast Track Designation, the FDA must determine, based on the request of a sponsor, that a product is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address an unmet medical need. The FDA will determine that a product will fill an unmet medical need if it will provide a therapy where none exists or provide a therapy that may be potentially superior to existing therapy based on efficacy or safety factors. The FDA may review sections of the NDA for a fast track product on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA.
The FDA may give a priority review designation to drugs that offer major advances in treatment, or provide a treatment where no adequate therapy exists. A priority review means that the goal for the FDA to review an application is six months, rather than the standard review of ten months under current PDUFA guidelines. Under the new PDUFA agreement, these six and ten month review periods are measured from the “filing” date rather than the receipt date for NDAs for new molecular entities, which typically adds approximately two months to the timeline for review and decision from the date of submission. Most products that are eligible for Fast Track Designation are also likely to be considered appropriate to receive a priority review.
29
In addition, products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may be eligible for accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require a sponsor of a drug receiving accelerated approval to perform post-marketing studies to verify and describe the predicted effect on irreversible morbidity or mortality or other clinical endpoint, and the drug may be subject to accelerated withdrawal procedures.
Moreover, under the provisions of the FDASIA, a sponsor can request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs designated as breakthrough therapies are also eligible for accelerated approval. The FDA must take certain actions, such as holding timely meetings and providing advice, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. We may explore some of these opportunities for our product candidates as appropriate.
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval. There also are continuing, annual user program fee requirements for any marketed products.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP requirements and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval of a drug or medical device is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in mandatory revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
|
|
• |
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
|
|
• |
fines, warning letters or holds on post-approval clinical trials; |
30
|
|
|
|
• |
refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product approvals; |
|
|
• |
product seizure or detention, or refusal to permit the import or export of products; or |
|
|
• |
injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs or devices may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
The Hatch-Waxman Amendments
The Drug Price Competition and Patent Term Restoration Act of 1984, known as the Hatch-Waxman Act, added two pathways for FDA drug approval. First, the Hatch-Waxman amendments authorized the FDA to approve an alternative type of NDA under Section 505(b)(2) of the FDCA. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from trials not conducted by or for the applicant and for which the applicant has not obtained a right of reference from the data owner. The applicant may rely upon the FDA’s findings of safety and efficacy for an approved product that acts as the “listed drug.” The FDA may also require 505(b)(2) applicants to perform additional studies or measurements to support the change from the listed drug. The FDA may then approve the new product candidate for all, or some, of the label indications for which the branded reference drug has been approved, as well as for any new indication sought by the 505(b)(2) applicant.
Second, the Hatch-Waxman amendments to the FDCA also established a statutory procedure for submission and FDA review and approval of abbreviated new drug applications, or ANDAs, for generic versions of branded drugs previously approved by the FDA (such previously approved drugs are referred to as “listed drugs”). An ANDA is a comprehensive submission that contains, among other things, data and information pertaining to the active pharmaceutical ingredient, drug product formulation, specifications and stability of the generic drug, as well as analytical methods, manufacturing process validation data and quality control procedures. Premarket applications for generic drugs are termed abbreviated because they generally do not include pre-clinical and clinical data to demonstrate safety and effectiveness. However, a generic manufacturer is typically required to conduct bioequivalence studies of its test product against the listed drug. The bioequivalence studies for orally administered, systemically available drug products assess the rate and extent to which the API is absorbed into the bloodstream from the drug product and becomes available at the site of action. Bioequivalence is established when there is an absence of a significant difference in the rate and extent for absorption of the generic product and the listed drug. For some drugs, other means of demonstrating bioequivalence may be required by the FDA, especially where rate and/or extent of absorption are difficult or impossible to measure. The FDA will approve the generic product as suitable for an ANDA application if it finds that the generic product does not raise new questions of safety and effectiveness as compared to the innovator product. A product is not eligible for ANDA approval if the FDA determines that it is not bioequivalent to the referenced innovator drug, if it is intended for a different use, or if it is not subject to an approved Suitability Petition.
In seeking approval for a drug through an NDA, including a 505(b)(2) NDA, applicants are required to list with the FDA certain patents whose claims cover the applicant’s product. Upon approval of an NDA, each of the patents listed in the application for the drug is then published in the Orange Book. Any applicant who files an ANDA seeking approval of a generic equivalent version of a drug listed in the Orange Book or a 505(b)(2) NDA referencing a drug listed in the Orange Book must certify to the FDA that (1) no patent information on the drug product that is the subject of the application has been submitted to the FDA; (2) such patent has expired; (3) the date on which such patent expires; or (4) such patent is invalid or will not be infringed upon by the manufacture, use or sale of the drug product for which the application is submitted. This last certification is known as a paragraph IV certification. A notice of the paragraph IV certification must be provided to each owner of the patent that is the subject of the certification and to the holder of the approved NDA to which the ANDA or 505(b)(2) application refers. The applicant may also elect to submit a “section viii” statement certifying that its proposed label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent.
31
If the reference NDA holder and patent owners assert a patent challenge directed to one of the Orange Book listed patents within 45 days of the receipt of the paragraph IV certification notice, the FDA is prohibited from approving the application until the earlier of 30 months from the receipt of the paragraph IV certification expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the applicant. The ANDA or 505(b)(2) application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the branded reference drug has expired.
Marketing Exclusivity
Market exclusivity provisions under the FDCA can delay the submission or the approval of certain marketing applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to obtain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not approve or even accept for review an abbreviated new drug application, or ANDA, or a NDA submitted under Section 505(b)(2), or 505(b)(2) NDA, submitted by another company for another drug based on the same active moiety, regardless of whether the drug is intended for the same indication as the original innovative drug or for another indication, where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement to one of the patents listed with the FDA by the innovator NDA holder. The FDCA alternatively provides three years of marketing exclusivity for an NDA, or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the modification for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for drugs containing the active agent for the original indication or condition of use. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the pre-clinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness. Pediatric exclusivity is another type of marketing exclusivity available in the United States. Pediatric exclusivity provides for an additional six months of marketing exclusivity attached to another period of exclusivity if a sponsor conducts clinical trials in children in response to a written request from the FDA. The issuance of a written request does not require the sponsor to undertake the described clinical trials. In addition, orphan drug exclusivity, as described above, may offer a seven-year period of marketing exclusivity, except in certain circumstances.
U.S. Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any therapeutic product candidate for which we may seek regulatory approval. Sales in the United States will depend in part on the availability of adequate financial coverage and reimbursement from third-party payors, which include government health programs such as Medicare, Medicaid, TRICARE and the Veterans Administration, as well as managed care organizations and private health insurers. Prices at which we or our customers seek reimbursement for our therapeutic product candidates can be subject to challenge, reduction or denial by payors.
The process for determining whether a payor will provide coverage for a product is typically separate from the process for setting the reimbursement rate that the payor will pay for the product. A payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be available. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. In order to obtain coverage and reimbursement for any product that might be approved for marketing, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of any products, which would be in addition to the costs expended to obtain regulatory approvals. Third-party payors may not consider our product candidates to be medically necessary or cost-effective compared to other available therapies, or the rebate percentages required to secure favorable coverage may not yield an adequate margin over cost or may not enable us to maintain price levels sufficient to realize an appropriate return on our investment in drug development.
32
Healthcare Reform
In the United States and some foreign jurisdictions, there have been, and continue to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of drug product candidates, restrict or regulate post-approval activities, and affect the profitable sale of drug product candidates.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, the ACA was passed, which substantially changed the way healthcare is financed by both the government and private insurers, and significantly impacts the U.S. pharmaceutical industry. The ACA, among other things: (i) increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extends the rebate program to individuals enrolled in Medicaid managed care organizations; (ii) established an annual, nondeductible fee on any entity that manufactures or imports certain specified branded prescription drugs and biologic agents apportioned among these entities according to their market share in some government healthcare programs; (iii) expanded the availability of lower pricing under the 340B drug pricing program by adding new entities to the program; (iv) increased the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; (v) expanded the eligibility criteria for Medicaid programs; (vi) created a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and (vii) established a Center for Medicare Innovation at CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drugs.
Some of the provisions of the ACA have yet to be implemented, and there have been judicial and Congressional challenges to certain aspects of the ACA, as well as recent efforts by the Trump administration to repeal or replace certain aspects of the ACA. While Congress has not passed comprehensive repeal legislation, bills affecting the implementation of certain taxes under the ACA have been signed into law. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain ACA-mandated fees, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device excise tax on non-exempt medical devices.
Other legislative changes have been proposed and adopted since the ACA was enacted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year and reduced payments to several types of Medicare providers. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. At the federal level, the Trump administration’s budget proposal for fiscal year 2019 contains further drug price control measures. While any proposed measures will require authorization through additional legislation to become effective, Congress and the Trump administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
33
U.S. Healthcare Fraud and Abuse Laws and Compliance Requirements
Federal and state healthcare laws and regulations restrict business practices in the pharmaceutical industry. The U.S. laws that may affect our ability to operate include:
|
|
• |
the federal Anti-Kickback Statute, which prohibits, among other things, persons from soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
|
|
• |
the federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent; |
|
|
• |
HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
|
|
• |
HIPAA, as amended by the federal Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; |
|
|
• |
the federal Physician Payments Sunshine Act, which among other things requires certain manufacturers of drugs, devices, and biologics, that are reimbursable by a federal healthcare program to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals, and ownership and investment interests held by physicians and their immediate family members; and |
|
|
• |
similar federal laws and state law equivalents of each of the above federal laws. |
Regulation Outside the United States
To the extent that any of our product candidates, once approved, are sold in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws and implementation of corporate compliance programs and reporting of payments or other transfers of value to healthcare professionals.
In order to market our future products in the EEA and many other foreign jurisdictions, we must obtain separate regulatory approvals. More concretely, in the EEA, medicinal products can only be commercialized after obtaining a Marketing Authorization, or MA. There are two types of marketing authorizations:
|
|
• |
the Community MA, which is issued by the European Commission through the Centralized Procedure, based on the opinion of the Committee for Medicinal Products for Human Use of the EMA and which is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of products, such as biotechnology medicinal products, orphan medicinal products, advanced therapy products, and medicinal products containing a new active substance indicated for the treatment certain diseases, such as AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU; and |
|
|
• |
National MAs, which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketing in a Member State of the EEA, this National MA can be recognized in another Member State through the Mutual Recognition Procedure. If the product has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. |
34
Under the above described procedures, before granting the MA, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
Data and Marketing Exclusivity
In the EEA, new products authorized for marketing, or reference products, qualify for eight years of data exclusivity and an additional two years of market exclusivity upon marketing authorization. The data exclusivity period prevents generic or biosimilar applicants from relying on the pre-clinical and clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar marketing authorization in the EU during a period of eight years from the date on which the reference product was first authorized in the EU. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the EU until 10 years have elapsed from the initial authorization of the reference product in the EU. The 10-year market exclusivity period can be extended to a maximum of eleven years if, during the first eight years of those 10 years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. In Japan, medicinal products approved for administration to a patient via a new route of administration qualify for six years of market exclusivity.
Clinical Trials
Clinical trials of medicinal products in the European Union must be conducted in accordance with European Union and national regulations and the International Conference on Harmonization, or ICH, guidelines on GCPs. Additional GCP guidelines from the European Commission, focusing in particular on traceability, apply to clinical trials of advanced therapy medicinal products. If the sponsor of the clinical trial is not established within the European Union, it must appoint an entity within the European Union to act as its legal representative. The sponsor must take out a clinical trial insurance policy, and in most EU countries, the sponsor is liable to provide “no fault” compensation to any study subject injured in the clinical trial.
Prior to commencing a clinical trial, the sponsor must obtain a clinical trial authorization from the competent authority, and a positive opinion from an IEC. The application for a clinical trial authorization must include, among other things, a copy of the trial protocol and an investigational medicinal product dossier containing information about the manufacture and quality of the medicinal product under investigation. Currently, clinical trial authorization applications must be submitted to the competent authority in each EU Member State in which the trial will be conducted. Under the new Regulation on Clinical Trials, which is currently expected to take effect in 2019, there will be a centralized application procedure where one national authority takes the lead in reviewing the application and the other national authorities have only a limited involvement. Any substantial changes to the trial protocol or other information submitted with the clinical trial applications must be notified to or approved by the relevant competent authorities and ethics committees. Medicines used in clinical trials must be manufactured in accordance with cGMP. Other national and European Union-wide regulatory requirements also apply.
Recycling
We started our business in 1976 as a used appliance retailer that reconditioned old appliances to sell in our stores. Under contracts with national and regional retailers of new appliances, such as Sears Roebuck and Co. and Montgomery Ward Inc., we collected the replaced appliance from the retailer’s customer’s residence when one of their stores delivered a new appliance in the Minneapolis/St. Paul, Miami, or Atlanta market. Any old appliances that we could not sell in our stores were sold to scrap metal processors. In the late 1980s, stricter environmental regulations began to affect the disposal of unwanted appliances and we were no longer able to take appliances that contained hazardous components to a scrap metal processor. At that time, we began to develop systems and equipment to remove the harmful materials so that metal processors would accept the appliance shells for processing. We then offered our services for disposing of appliances in an environmentally sound manner to appliance manufacturers and retailers, waste hauling companies, rental property managers, local governments, and the public.
35
In 1989, we began contracting with electric utility companies to provide turnkey appliance recycling services to support their energy conservation efforts. Since that time, we have provided our services to approximately 400 utilities and other providers of energy efficiency programs throughout North America.
We currently have contracts to recycle, or to replace and recycle, appliances for approximately 180 utilities across North America.
We have seen continued interest from sponsors of energy efficiency initiatives that recognize the effectiveness of recycling and replacing energy inefficient appliances. We are aggressively pursuing electric, water, and gas utilities, public housing authorities, and energy efficiency management companies going forward and expect that we will continue to submit proposals for various new appliance recycling and replacement programs accordingly. However, for a variety of reasons, we still have a limited ability to project revenues from utility programs. We cannot predict recycling volumes or if we will be successful in obtaining new contracts in the next fiscal year.
We operate 13 recycling centers in the U.S. and Canada to process and recycle old appliances according to all federal, state, provincial, and local rules and regulations. ARCA uses U.S. EPA RAD-compliant methods to remove and properly manage hazardous components and materials, including CFC refrigerants, mercury, polyurethane foam insulation and recyclable materials, such as ferrous and nonferrous metals, plastics, and glass. All of our facilities comply with licensing and permitting requirements, and employees who process appliances receive extensive safety and hazardous materials training.
Major household appliances in the United States include:
|
Refrigerators |
|
Clothes washers |
|
Freezers |
|
Clothes dryers |
|
Ranges/ovens |
|
Room air conditioners |
|
Dishwashers |
|
Dehumidifiers |
|
Microwave ovens |
|
Humidifiers |
Improper disposal of old appliances threatens air, ground, and water resources because many types of major appliances contain substances that can damage the environment. These harmful materials include:
|
|
1. |
Mercury, which easily enters the body through absorption, inhalation, or ingestion, potentially causing neurological damage. Mercury-containing components may be found in freezers, washers, and ranges. |
|
|
2. |
Chlorofluorocarbon (“CFC”), hydrochlorofluorocarbon, and hydrofluorocarbon refrigerants (collectively, “Refrigerants”), which cause long-term damage to the earth’s ozone layer and may contribute to global climate change. Refrigerators, freezers, room air conditioners, and dehumidifiers commonly contain Refrigerants. |
|
|
3. |
CFCs, having a very high ozone-depletion potential that may also be used as blowing agents in the polyurethane foam insulation of refrigerators and freezers. |
|
|
4. |
Other materials, such as oil, that are harmful when released into the environment. |
The U.S. federal government requires the recovery of Refrigerants upon appliance disposal and also regulates the management of hazardous materials found in appliances. Most state and local governments have also enacted laws affecting how their residents dispose of unwanted appliances. For example, many areas restrict landfills and scrap metal processors from accepting appliances unless the units have been processed to remove environmentally harmful materials. As a result, old appliances usually cannot be discarded directly through ordinary solid waste systems.
In addition to these solid waste management and environmental issues, energy conservation is another compelling reason for proper disposal of old appliances. The U.S. Department of Energy’s updated appliance energy efficiency standards that took effect in September 2014 require new refrigerators to be 25-to-30% more efficient than those manufactured only one year earlier. Refrigerators manufactured today use about one-fifth as much electricity as units made in the mid-1970s.
36
While new refrigerators can save a significant amount of energy in the home, more than 30% of all U.S. households have a second refrigerator in the basement or garage. These units are typically 15-to-25 years old and consume about 750 to 1500 kilowatt-hours per year, driving electric bills up by more than $150 annually per household.
Utilities have become important participants in dealing with energy inefficient appliances as a way of reducing peak demand on their systems and avoiding the capital and environmental costs of adding new generating capacity. To encourage the permanent removal of energy inefficient appliances from use, many electric utility companies sponsor programs through which their residential customers can retire working refrigerators, freezers, and room air conditioners. Utility companies often provide assistance and incentives for consumers to discontinue use of a surplus appliance or to replace their old, inefficient appliances with newer, more efficient models. To help accomplish this, some utilities offer appliance replacement programs for some segments of their customers, through which older model kitchen and laundry appliances are recycled and new highly efficient ENERGY STAR® units are installed.
The U.S. Environmental Protection Agency (the “EPA”) has been supportive of efforts by electric utilities and other entities that sponsor appliance recycling programs to ensure that the collected units are managed in an environmentally sound manner. In October 2006, the EPA launched the Responsible Appliance Disposal (“RAD”) Program, a voluntary partnership program designed to help protect the ozone layer and reduce emissions of greenhouse gases. Through the program, RAD partners use best practices to recover ozone-depleting chemicals and other harmful materials from old refrigerators, freezers, room air conditioners, and dehumidifiers. Because of our appliance recycling expertise, we were active participants in helping to design the RAD program and currently submit annual reports to the EPA to document the environmental benefits our utility customers that are RAD partners have achieved through their recycling programs.
In October 2009, we entered into a Joint Venture Agreement (the “Joint Venture Agreement”) with 4301 Operations, LLC (“4301”) to establish and operate a regional processing center (“RPC”). At the time of the formation of this joint venture, we believed that 4301 had significant experience in the recycling of major household appliances and, in connection therewith, 4301 contributed its then-existing business and equipment to the joint venture. Under the Joint Venture Agreement, the parties formed a new entity known as ARCA Advanced Processing, LLC (“AAP”), in which each party had a 50% interest. In connection with the formation of the joint venture, we contributed $2.0 million to the joint venture. The joint venture commenced operations on February 8, 2010. On August 15, 2017, ARCA entered into an Equity Purchase Agreement with 4301 and sold its 50% joint venture interest in AAP to 4301 in consideration of $800,000 in cash. The gain recorded by ARCA was $81,000. On the same date and in a separate, related transaction, ARCA entered into an Asset Purchase Agreement with Recleim PA, LLC (“Recleim”), and the other parties thereto. Under the agreement, ARCA agreed to license certain intellectual property under patent No. 8,931,289 to Recleim for use at 4301 North Delaware Avenue, Philadelphia, Pennsylvania or any successor facility within 15 miles of where Recleim conducts business. On August 15, 2017, Recleim (i) paid in full all AAP indebtedness owed to BB&T Bank in the amount of $3,454,000, (ii) terminated and released all security interests in AAP and ARCA’s equipment as part of Recleim’s purchase of certain equipment and assets from AAP on the same date, and (iii) assumed approximately $768,000 in AAP liabilities and all of ARCA’s liabilities to Haier US Appliance Solutions, Inc., dba GE Appliances.
Our wholly-owned subsidiaries in our Recycling segment include, ARCA Canada Inc., a Canadian corporation formed in September 2006, ARCA Recycling, Inc., a California corporation formed in November 1991, and Customer Connexx, LLC, a Nevada limited liability company formed in October 2016 that provides call center services for recycling business.
Technology
On August 18, 2017, in a move to diversify our offering beyond our then-current appliance recycling capabilities, the Company acquired GeoTraq by way of merger. As a result of this transaction, GeoTraq became a wholly-owned subsidiary of the Company. In connection with this transaction, the Company tendered to the three owners of GeoTraq $200,000, issued to them an aggregate of 288,588 shares of the Company’s Series A Convertible Preferred Stock then valued at $14,963,288 inclusive of the beneficial conversion feature, and issued three separate one-year unsecured promissory notes for an aggregate original principal amount of $800,000. These unsecured promissory notes have been repaid in full. In addition, there was $10,133,366 deferred tax liability associated with the purchase of the intangible assets of GeoTraq. The total value of the intangible assets purchased was $26,096,654, including the deferred tax liability.
37
GeoTraq is a Mobile Internet of Things (“IoT”) technology company that designs innovative wireless modules that provide Location Based Services (“LBS”) and connect external sensors to the IoT. GeoTraq is planning to manufacture and sell wireless transceiver modules and subscription services that will allow connectivity using publicly available global Mobile IoT networks. GeoTraq addresses the large LBS market segment that is currently under served with existing solutions due to high deployment costs (hardware, service, logistics), limited battery life and large form factors. We believe that there is a large under-served portion of the LBS market that is not addressed by existing solutions. RFID and Wi-Fi require close proximity for asset tracking, while GPS is too bulky and power hungry for many needs. GeoTraq addresses the white space in-between by designing wireless transceiver modules with technology that provides LBS directly from global Mobile IoT networks. GeoTraq’s technology allows for a substantially lower cost solution, extended service life, a small form factor, and even disposable devices, which we believe can significantly reduce return logistics costs.
GeoTraq applied for and was granted Patent No. 10,182,402, which covers various aspects of operation of its Mobile IoT wireless modules. A description of the patent features includes:
|
|
1. |
An apparatus comprising: an interval timer; a power control; a Short Message Service (SMS) packetizer; a geo-locator; a radio frequency (RF) communicator; and a controller and a memory, the memory comprising instructions for the controller to operate the interval timer cooperatively with the power control to cause a transition of the geo-locator from a sleep state to a wake state after a preset defined time interval, and to operate the geo-locator to receive signal strength levels and corresponding cell IDs from a plurality of cellular base stations, and to operate the SMS packetizer to package the signal strength levels and the corresponding cell IDs into a first outgoing SMS message, and to communicate the first outgoing SMS message to a preset address using the RF communicator. |
|
|
2. |
The apparatus of claim 1, further comprising: a subscriber identity module (SIM); and the memory further comprising instructions to block visibility to the SIM by the geo-locator for a limited duration after the transition of the geo-locator from the sleep state to the wake state after the defined time interval. |
|
|
3. |
The apparatus of claim 2, further comprising: the memory further comprising instructions to override a preset floor on the signal strength levels during the limited duration after the transition of the geo-locator from the sleep state to the wake state after the defined time interval. |
|
|
4. |
The apparatus of claim 1, further comprising: the memory further comprising instructions to operate the SMS packetizer to package the signal strength levels with the corresponding cell IDs. |
|
|
5. |
The apparatus of claim 1, further comprising: the memory further comprising instructions to receive a command SMS message via the RF communicator; a parser to extract a time interval command from the received command SMS message; and the memory further comprising instructions to apply the time interval command to the interval timer to set the defined time interval. |
|
|
6. |
The apparatus of claim 1, further comprising: the memory further comprising instructions to receive a response SMS message via the RF communicator, the response SMS message being a response to the first outgoing SMS message; a parser to extract geo-locations for cell IDs from the response SMS message; and the memory further comprising instructions to associate the geo-locations for each of the cell IDs from the response message with corresponding cell IDs in the memory. |
|
|
7. |
A method comprising: applying an interval timer to a power control to control power for a subscriber identify module (SIM), a SMS packetizer, a geo-locator, and a radio frequency (RF) communicator after a preset defined time interval; operating the interval timer cooperatively with the power control to cause a transition of the geo-locator from a sleep state to a wake state after the defined time interval; operating the geo-locator to receive signal strength levels and corresponding cell ids from a plurality of cellular base stations; operating the SMS packetizer to package the signal strength levels and the corresponding cell IDs into an outgoing SMS message; and communicating the outgoing SMS message to a preset address using the RF communicator. |
|
|
8. |
The method of claim 7, further comprising: blocking visibility to the SIM by the geo-locator for a limited duration after the transition. |
|
|
9. |
The method of claim 8, further comprising: overriding a preset floor on the signal strength levels during the limited duration after the transition. |
38
|
|
10. |
The method of claim 7, further comprising: receiving a command SMS message via the RF communicator; extracting a time interval command from the command SMS message; and applying the time interval command to the interval timer to set the defined time interval. |
|
|
11. |
The method of claim 7, further comprising: receiving a response SMS message via the RF communicator in response to the outgoing SMS message; extracting geo-locations for cell IDs from the response SMS message; and associating the geo-locations for each of the cell ids from the response SMS message with corresponding cell IDs in a memory. |
With the GeoTraq acquisition, we expect to have the ability to deploy IoT devices to locate, monitor and track the movement of inventory and other assets and monitor connected sensors. Our GeoTraq subsidiary has not generated any revenue to date, including in the fiscal year ended December 28, 2019.
ApplianceSmart, Inc.
Prior to December 30, 2017, we sold new and out-of-the-box major household appliances in the United States though a chain of Company-owned retail stores operating under the name ApplianceSmart®. On December 30, 2017, we, together with our then-subsidiary, ApplianceSmart, Inc. (“ApplianceSmart”), entered into a Stock Purchase Agreement (the “Stock Purchase Agreement”) with ApplianceSmart Holdings LLC (the “Purchaser”), a wholly-owned subsidiary of Live Ventures Incorporated (Nasdaq: Live), pursuant to which we sold to the Purchaser all of the issued and outstanding shares of capital stock of ApplianceSmart (the “ApplianceSmart Stock”) in exchange for $6.5 million. Effective April 1, 2018, the Purchaser issued the Company a promissory note (the “ApplianceSmart Note”) with a three-year term in the original principal amount of $3.9 million for the balance of the purchase price. ApplianceSmart is guaranteeing the repayment of the ApplianceSmart Note. On December 26, 2018, the ApplianceSmart Note was amended and restated to grant ARCA a security interest in the assets of the Purchaser, ApplianceSmart, and ApplianceSmart Contracting Inc. in exchange for modifying the repayment terms to provide for the payment in full of all accrued interest and principal on April 1, 2021, the maturity date of the ApplianceSmart Note. On March 15, 2019, JanOne entered into subordination agreements with third parties, pursuant to which it agreed to subordinate the payment of indebtedness under the ApplianceSmart Note and its security interest in the assets of ApplianceSmart and other related parties in exchange for receipt of a payment of up to $1.2 million within 15 days of the subordination agreement. On December 9, 2019, ApplianceSmart filed a voluntary petition (the “Chapter 11 Case”) in the United States Bankruptcy Court for the Southern District of New York (the “Bankruptcy Court”), seeking relief under Chapter 11 of Title 11 of the United States Code (the “Bankruptcy Code”). As of December 28, 2019, indebtedness owed by ApplianceSmart to JanOne is approximately $2.9 million. However, JanOne has recorded a full valuation allowance for the entire amount of the indebtedness due to the uncertainty of repayment.
Customers and Source of Supply for Recycling and Technology
Recycling: We contract with utility companies or their program administrators and other sponsors of energy efficiency programs to provide a full range of appliance recycling and replacement services to help them achieve their energy savings goals. The contracts usually have terms of one-to-three years, with provisions for renewal at the option of the utility. Under some contracts, we manage all aspects, including advertising of the appliance recycling or replacement program. Under other contracts, we provide only specified services, such as collection and recycling.
Our contracts with utility customers prohibit us from repairing and selling appliances or appliance parts we receive through their programs. We have instituted tracking and auditing procedures to assure our customers that those appliances do not return to use.
Our pricing for energy efficiency program contracts is generally on a per-appliance basis and depends upon several factors, including:
|
|
1. |
Total number of appliances expected to be processed and/or replaced. |
|
|
2. |
Length of the contract term. |
|
|
3. |
Specific services the utility requires us to provide. |
|
|
4. |
Market factors, including labor rates and transportation costs. |
39
|
|
|
|
5. |
Anticipated revenue associated with the sale of recycled appliance byproducts. |
|
|
6. |
Competitive bidding scenarios. |
GeoTraq: GeoTraq currently has no customers. GeoTraq sources its raw materials, including electronic chips, computers, and software from various third parties. GeoTraq is dependent on a single supplier for its modules.
Principal Products and Services for Recycling and Technology
At December 28, 2019, we generated revenues from two sources: recycling and byproducts. Recycling revenues were generated by charging fees for collecting and recycling appliances for utilities and other sponsors of energy efficiency programs and through the sale of new ENERGY STAR® appliances to utility companies for installation in the homes of a specific segment of their customers. Byproduct revenues were generated by selling scrap materials, such as metal and plastics, from appliances we collected and recycled.
During fiscal year 2019, we operated three reportable segments: biotechnology, recycling, and technology. During fiscal year 2018, we operated two reportable segments: recycling and technology (commencing on August 18, 2017). Our recycling segment includes all fees charged for collecting, recycling, and installing appliances for utilities and other customers and includes byproduct revenue, which is generated primarily through the recycling of appliances. Our technology segment is engaged in the development, design, and ultimately, we expect, the sale of cellular transceiver modules, also known as Mobile IoT modules.
Seasonality for Recycling and Technology
Promotional activities for programs in which the utility sponsor conducts all advertising are generally strong during the second and third calendar quarters, leading to higher customer demand for services during that time period. As a result, we experience a surge in business during the second and third calendar quarters, which generally declines through the fourth and first calendar quarters until advertising activities resume.
Our technology segment did not have any customers at December 28, 2019.
Competition for Recycling and Technology
Recycling:
Many factors, including obtaining adequate resources to create and support the infrastructure required to operate large-scale appliance recycling and replacement programs, affect competition in the industry. We generally compete for contracts with several other appliance recycling businesses, energy services management companies, and new-appliance retailers. We also compete with small hauling or recycling companies that are based in the program’s service territory. Many of these companies, including used-appliance dealers that call themselves “appliance recyclers,” resell in the secondary market a percentage of the used appliances they accept for recycling. The unsalable units may not be properly processed to remove environmentally harmful materials because these companies do not have the capability to offer the full range of services we provide.
We expect our primary competition for appliance recycling and replacement contracts with existing and new customers to come from a variety of sources, including:
|
|
1. |
Existing recycling companies. |
|
|
2. |
Entrepreneurs entering the appliance recycling business. |
|
|
3. |
Management consultants. |
|
|
4. |
Major waste hauling companies. |
|
|
5. |
Scrap metal processors. |
|
|
6. |
National and regional new appliance retailers. |
40
|
|
In addition, utility companies and other customers may choose to provide all or some of the services required to operate their appliance recycling and replacement programs internally rather than contracting with outside vendors. We have no assurance that we will be able to compete profitably in any of our chosen markets.
Technology
GeoTraq plans on operating in an industry segment that is made of numerous competing technologies designed to connect devices to the IoT. The business’s wireless solution uses IoT based on LTE CAT-M and the newly released NB-IoT protocols that were defined in the GSMA’s (Groupe Speciale Mobile Association) 3GPP Release 13 standard. The Mobile IoT industry utilizes radio spectrum that is licensed to wireless carries by various governmental regulatory agencies around the world. Mobile IoT is extremely competitive and constantly changing as carriers, manufacturers, and solution providers offer innovation to the IoT marketplace. GeoTraq believes there is a large under-served opportunity for “Simple IoT” solutions that significantly reduce the complexity, cycle time and cost of deploying LBS and sensor monitoring solutions. The company’s transceiver modules and associated wireless connectivity subscription service is specifically targeted at accomplishing these objectives.
Government Regulation for Recycling and Technology
Recycling
Federal, state, and local governments regulate appliance collection, recycling, and sales activities. While some requirements apply nationwide, others vary by market. The many laws and regulations that affect appliance recycling include landfill disposal restrictions, hazardous waste management requirements, and air quality standards. For example, the 1990 Amendments to the Clean Air Act prohibit the venting of all Refrigerants while servicing or disposing of appliances.
Each of our recycling facilities maintains the appropriate registrations, permits, and licenses for operating at its location. We register our recycling centers as hazardous waste generators with the EPA and obtain all appropriate regional and local licenses for managing hazardous wastes. Licensed hazardous waste companies transport and recycle or dispose of the hazardous materials we generate. Our collection vehicles and our transportation employees are required to comply with all U.S. Department of Transportation (“DOT”) licensing requirements.
Approximately 30 of ARCA Recycling’s clients participate in the EPA’s voluntary RAD program by committing to employ best environmental practices to reduce emissions of ozone-depleting substances and greenhouse gases through the proper disposal of refrigeration appliances at end of life. We prepare annual RAD reports that quantify the materials collected to submit to EPA on behalf of our clients.
Although we believe that further governmental regulation of the appliance recycling industry could have a positive effect on us, we cannot predict the direction of future legislation. Under some circumstances, for example, further regulation could materially increase our operational costs or reduce environmental requirements for disposing of appliances at end of life. In addition, under some circumstances we may be subject to contingent liabilities because we handle hazardous materials. We believe we are in compliance with all government regulations regarding the handling of hazardous materials, and we have environmental insurance to mitigate the impact of any potential contingent liability.
Technology
GeoTraq’s Mobile IoT modules utilize low-power wireless transmitters that emit RF energy waves, which are subject to regulation by the Federal Communications Commission (“FCC”) and may be subject to regulation by other domestic and international agencies. GeoTraq believes that FCC rules Part 15, Part 20, Part 22, Part 24, and Part 27 may apply to the company’s products. GeoTraq believes that its products are safe and will utilize FCC accredited testing laboratories to verify and certify that its modules comply with all required regulatory requirements. In addition, GeoTraq intends to seek and obtain necessary licenses and permits from the FCC and other regulatory agencies as required by law.
41
SELECTED CONSOLIDATED FINANCIAL DATA
The following tables set forth selected consolidated financial data for the periods ended or as of the dates indicated. Such historical consolidated financial data should be read in conjunction with the information set forth in our Annual Report on Form 10-K for the year ended December 28, 2019, filed with the SEC on April 6, 2020 and incorporated herein by reference.
The statement of operations data presented below for each of the years ended December 28, 2019 and December 29, 2018, and the balance sheet data as of December 28, 2019 and December 29, 2018, are derived from the audited “Consolidated Financial Statements” contained in our Annual Report on Form 10-K for the year ended December 28, 2019. Our historical results are not necessarily indicative of the results to be expected for any future periods.
(in thousands, except for loss per share)
|
|
|
For the 52-Week Period Ended |
|
|||||
|
Statement of Operations Data |
|
December 28, 2019 |
|
|
December 29, 2018 |
|
||
|
Revenues |
|
$ |
35,097 |
|
|
$ |
36,794 |
|
|
Cost of revenues |
|
|
27,311 |
|
|
|
25,741 |
|
|
Gross profit |
|
|
7,786 |
|
|
|
11,053 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Selling, general and administrative expenses |
|
|
20,217 |
|
|
|
17,150 |
|
|
Operating loss |
|
|
(12,431 |
) |
|
|
(6,097 |
) |
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
(1,480 |
) |
|
|
(668 |
) |
|
Impairment charges |
|
|
(2,992 |
) |
|
|
— |
|
|
Gain on litigation settlement |
|
|
694 |
|
|
|
— |
|
|
Other income, net |
|
|
1,048 |
|
|
|
430 |
|
|
Total other expense, net |
|
|
(2,730 |
) |
|
|
(238 |
) |
|
Loss from operations before benefit from income taxes |
|
|
(15,161 |
) |
|
|
(6,335 |
) |
|
Income tax benefit |
|
|
3,197 |
|
|
|
727 |
|
|
Net loss |
|
$ |
(11,964 |
) |
|
$ |
(5,608 |
) |
|
Loss per share: |
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share |
|
$ |
(6.78 |
) |
|
$ |
(3.75 |
) |
|
Weighted average common shares outstanding: |
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
1,763,670 |
|
|
|
1,494,941 |
|
|
|
|
December 28, 2019 |
|
|
December 29, 2018 |
|
||
|
Balance Sheet Data |
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
29,034 |
|
|
$ |
35,040 |
|
|
Current liabilities |
|
|
17,573 |
|
|
|
9,684 |
|
|
Long term liabilities |
|
|
1,120 |
|
|
|
3,745 |
|
|
Total stockholders' equity |
|
|
10,341 |
|
|
|
21,611 |
|
42
DESCRIPTION OF SECURITIES WE MAY OFFER
We may issue from time to time, in one or more offerings the following securities:
|
• |
shares of Common Stock; |
|
• |
shares of Preferred Stock, which may be convertible into shares of Common Stock; |
|
• |
debt securities, which may be senior or subordinated and may be convertible into or exchangeable for shares of Common Stock or shares of Preferred Stock; |
|
• |
warrants exercisable for debt securities, shares of Common Stock, or shares of Preferred Stock; |
|
• |
rights to purchase any of such securities; and |
|
• |
units composed of our debt securities, shares of Common Stock, shares of Preferred Stock, and warrants, in any combination. |
This prospectus contains a summary of the material general terms of the various securities that we may offer. The specific terms of the securities will be described in a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, which may be in addition to or different from the general terms summarized in this prospectus. Where applicable, the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials will also describe any material United States federal income tax considerations relating to the securities offered and indicate whether the securities offered are or will be listed on any securities exchange. The summaries contained in this prospectus and in any prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, may not contain all of the information that you would find useful. Accordingly, you should read the actual documents relating to any securities sold pursuant to this prospectus. See “Available Information” and “Incorporation of Certain Information by Reference” for information about how to obtain copies of those documents.
The terms of any particular offering, the initial offering price, and the net proceeds to us will be contained in the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, relating to such offering.
43
The following summary of terms of our Common Stock and our Preferred Stock is based upon our Articles of Incorporation (our “Charter”) and Bylaws (our “Bylaws”), currently in effect, and under Chapter 78 of the Nevada Revised Statutes (the “NRS”). This summary is not complete and is subject to, and qualified in its entirety by reference to, our Charter and our Bylaws. For a complete description of the terms and provisions of our Common Stock, please refer to our Charter and Bylaws, which are filed as exhibits to Registration Statement of which this prospectus forms a part. Throughout this section, references to “we,” “our,” and “us” refer to JanOne Inc. and its subsidiaries. We encourage you to carefully read these documents and the applicable provisions of the NRS.
General
Our authorized capital stock consists of 10,000,000 shares of Common Stock and 2,000,000 shares of Preferred Stock, of which 259,729 shares are designated as Series A-1 Convertible Preferred Stock, par value $0.001 per share.
As of December 15, 2020, we had 1,829,982 shares of our Common Stock issued and outstanding and 259,729 shares of our Series A-1 Preferred Stock issued and outstanding.
The authorized and unissued shares of Common Stock and Preferred Stock are available for issuance without further action by our stockholders, unless such action is required by applicable law or the rules of any stock exchange on which our securities may then be listed. Unless approval of our stockholders is so required, our Board of Directors (our “Board”) does not currently intend to seek stockholder approval for the issuance and sale of our Common Stock.
All of our issued and outstanding shares of our capital stock are fully paid and non-assessable.
Common Stock
Voting, Dividend, and Liquidation Rights
Each holder of our Common Stock is entitled to one vote for each share issued and outstanding held on all matters to be voted upon by the stockholders. Our Charter does not provide for cumulative voting in the election of directors. Subject to the rights of the holders of the Series A-1 Preferred Stock to their preferential dividend in accordance with the provisions of our Charter, the holders of shares of our Common Stock and Series A-1 Preferred Stock (on an as-if-converted to Common Stock basis in accordance with the terms of our Charter) will be entitled to such cash dividends as may be declared from time to time by our Board from funds available therefor. Upon liquidation, dissolution, or winding up of the Company, and after all liquidation preferences payable to any series of Preferred Stock entitled thereto have been satisfied, our remaining assets shall be distributed to all holders of Common Stock and any similarly situated stockholders who are not entitled to any liquidation preference or, if there be an insufficient amount to pay all such stockholders, then ratably among such holders.
Preemptive or Other Rights
Our shares of Common Stock do not have any preemptive, conversion, or redemption rights.
Stockholder Action; Special Meetings
Stockholders’ actions can only be taken at an annual or special meeting of our stockholders. Our Bylaws provide that special meetings of the stockholders may be called at any time only by (i) our Chief Executive Officer, (ii) two of the members of the Board, or (iii) upon a written request of stockholders holding 10% or more of the capital stock entitled to vote.
Board of Directors; Removal; Vacancies
Our Bylaws specify that the number of directors is to be determined by a majority vote of the Board. Our Board is currently composed of five directors. We do not have a classified Board. Pursuant to our Bylaws and the NRS, a director serves until the regular meeting next following or closely coinciding with the expiration of his or her term of office and until his or her successor has been elected and qualified, or until his or her earlier death, removal, or resignation.
44
Limitation of Liability and Indemnification
Our Charter provides that none of our directors and officers shall be personally liable to us or our stockholders for damages for breach of fiduciary duty as a director or officer, except for liability for (i) acts or omissions that involve intentional misconduct, fraud, or knowing violation of law or (ii) for authorizing any distribution in violation of Section 78.300 of the NRS. Our Bylaws provide that any officer or director who is made a party or witness to an action, suit, or proceeding, whether civil, criminal, administrative, or investigative, by reason of the fact that he or she is or was one of our directors or officers or serving at our request as a director, officer, employee, or agent, shall be indemnified and held harmless by us to the fullest extent authorized by the NRS. The right to indemnification shall include the right of advancement of expenses to the extent permitted under the NRS.
Listing and Transfer Agent
Our Common Stock is listed on The Nasdaq Capital Market under the symbol “JAN.” The transfer agent and registrar for our Common Stock is EQ Shareowner Services.
Series A-1 Convertible Preferred Stock
In connection with the Company’s acquisition of GeoTraq, the Company issued to the then stockholders of GeoTraq, among other consideration, an aggregate of 288,588 shares of the Company’s Series A Convertible Preferred Stock (the “Series A Preferred Stock”). To accomplish the designation and issuance of the Series A Preferred Stock, we filed a Certificate of Designation with the Secretary of State of the State of Minnesota. On November 9, 2017, we filed a Certificate of Correction with the Minnesota Secretary of State. In connection with our reincorporation from the State of Minnesota to the State of Nevada in March 2018, we filed Articles of Incorporation with the Secretary of State of the State of Nevada on March 12, 2018, and a Certificate of Correction with the Secretary of State of the State of Nevada on August 7, 2018 (collectively, the “Nevada Articles of Incorporation”). On June 21, 2019, we filed a Certificate of Designation (the “Series A-1 Certificate of Designation”) of Powers, Preferences, and Rights of Series A-1 Convertible Preferred Stock (the “Series A-1 Preferred Stock”) with the Nevada Secretary of State. On October 1, 2020, we filed an Amended and Restated Certificate of Designation (the “Amended and Restated Series A-1 Certificate of Designation”) of Powers, Preferences, and Rights of Series A-1 Convertible Preferred Stock with the Nevada Secretary of State. The following summary of the Nevada Articles of Incorporation and Amended and Restated Series A-1 Certificate of Designation does not purport to be complete and is qualified in its entirety by reference to the provisions of applicable law and to the Nevada Articles of Incorporation and the Amended and Restated Series A-1 Certificate of Designation, which were initially filed as Exhibit 3.3 to the Company’s Current Report on Form 8-K, filed with the SEC on March 13, 2018, and as Exhibit 3.8(a) to the Company’s Current Report on Form 8-K, filed with the SEC on October 2, 2020, respectively.
The Series A-1 Preferred Stock was designated pursuant to guidance received from Nasdaq and has virtually all of the same rights, characteristics, and attributes as the Company’s Series A Preferred Stock, except as required by the Listing Qualifications staff of The Nasdaq Stock Market LLC (i.e., Section 3.2.5 in respect of voting rights of the Series A-1 Preferred Stock and Section 3.2.1(f) in respect of a Triggering Event, as such term is defined therein, and the formula to be applied in connection therewith), with respect to each of which requirements the Company has already been in compliance. The filing of the Series A-1 Certificate of Designation was unanimously approved by the Board of Directors on June 18, 2019. The affirmative approval of a majority of the holders of the Series A Preferred Stock for the exchange of such shares into shares of Series A-1 Preferred Stock occurred on or about June 19, 2019. The three holders of our Series A Preferred Stock were deemed to have exchanged their outstanding shares of Series A Preferred Stock for an equivalent number of shares of Series A-1 Preferred Stock, or an aggregate of 259,729 shares. The filing of the Amended and Restated Series A-1 Certificate of Designation was unanimously approved by the Board of Directors and by a majority of the holders of the Series A-1 Preferred Stock on October 1, 2020.
Dividends
We cannot declare, pay, or set aside any dividends on shares of any other class or series of our capital stock unless (in addition to the obtaining of any consents required by our Articles of Incorporation) the holders of the Series A-1 Preferred Stock then outstanding shall first receive, or simultaneously receive, a dividend in the aggregate amount of
45
$1.00, regardless of the number of then-issued and outstanding shares of Series A-1 Preferred Stock. Any remaining dividends allocated by the Board of Directors shall be distributed in an equal amount per share to the holders of outstanding Common Stock and Series A-1 Preferred Stock (on an as-if-converted to Common Stock basis pursuant to the Conversion Ratio as defined below).
Conversion
The shares of Series A-1 Preferred Stock have conversion rights into an aggregate of 85.0% of GeoTraq, Inc., currently a wholly-owned subsidiary of the Company.
Redemption
The shares of Series A-1 Preferred Stock have no redemption rights.
Preemptive Rights
Holders of shares of Series A-1 Preferred Stock are not entitled to any preemptive rights in respect to any securities of the Company, except as set forth in the Series A-1 Certificate of Designation or any other document agreed to by us.
Voting Rights
Each holder of a share of Series A-1 Preferred Stock has that number of votes as is determined by multiplying (i) the number of shares of Series A Preferred Stock held by such holder and (ii) 17. The holders of Series A-1 Preferred Stock vote together with all other classes and series of Common Stock and Preferred Stock of the Company as a single class on all actions to be taken by the holders of Common Stock of the Company, except to the extent that voting as a separate class or series is required by law.
Protective Provisions
Without first obtaining the affirmative approval of a majority of the holders of the shares of Series A-1 Preferred Stock, we may not directly or indirectly (i) increase or decrease (other than by redemption or conversion) the total number of authorized shares of Series A-1 Preferred Stock; (ii) effect an exchange, reclassification, or cancellation of all or a part of the Series A-1 Preferred Stock, but excluding a stock split or reverse stock split or combination of the Common Stock or preferred stock; (iii) effect an exchange, or create a right of exchange, of all or part of the shares of another class of shares into shares of Series A-1 Preferred Stock; or (iv) alter or change the rights, preferences, or privileges of the shares of Series A-1 Preferred Stock so as to affect adversely the shares of such series, including the rights set forth in the Series A-1 Certificate of Designation; provided, however, that we may, without any vote of the holders of shares of the Series A-1 Preferred Stock, make technical, corrective, administrative, or similar changes to the Series A-1 Certificate of Designation that do not, individually or in the aggregate, materially adversely affect the rights or preferences of the holders of shares of the Series A-1 Preferred Stock.
Anti-Takeover Effects of Certain Provisions of our Charter, our Bylaws, and the NRS
Certain provisions of the NRS and our Charter and Bylaws could make more difficult the acquisition of us by means of a tender offer or otherwise, and the removal of incumbent officers and directors. These provisions are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of us.
Business Combinations
The “business combination” provisions of Sections 78.411 to 78.444, inclusive, of the NRS prohibit a Nevada corporation with at least 200 stockholders (at least 100 of whom are stockholders of record and residents of the State of Nevada) from engaging in various “combination” transactions with any interested stockholder for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the
46
transaction is approved by the entity’s board of directors prior to the date the interested stockholder obtained such status; or after the expiration of the three-year period, unless:
|
|
• |
the transaction is approved by the entity’s board of directors or a majority of the voting power held by disinterested stockholders of the entity, or |
|
|
• |
if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the three years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher. |
A “combination” is defined to include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer, or other disposition, in one transaction or a series of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation, or (c) 10% or more of the earning power or net income of the corporation.
In general, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within three years, did own) 10% or more of an entity’s voting stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire us even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Acquisitions of Controlling Interest
Nevada’s “acquisition of controlling interest” statutes (NRS 78.378 through 78.3793, inclusive) contain provisions governing the acquisition of a controlling interest in certain Nevada corporations. These “control share” laws provide generally that any person who acquires a “controlling interest” in certain Nevada corporations may be denied voting rights, unless a majority of the disinterested stockholders of the corporation elects to restore such voting rights. These laws would apply to us as of a particular date if we were to have 200 or more stockholders of record (at least 100 of whom have addresses in Nevada appearing on our stock ledger at all times during the 90 days immediately preceding that date) and do business in the State of Nevada directly or through an affiliated corporation, unless our Charter or Bylaws in effect on the tenth day after the acquisition of a controlling interest provide otherwise. These laws provide that a person acquires a “controlling interest” whenever a person acquires shares of a subject corporation that, but for the application of these provisions of the NRS, would enable that person to exercise (1) one-fifth or more, but less than one-third, (2) one-third or more, but less than a majority, or (3) a majority or more of all of the voting power of that corporation in the election of its directors. Once an acquirer crosses one of these thresholds, shares that it acquired in the transaction that took it over the threshold and shares that it acquired within the 90 days immediately preceding the date when it acquired or offered to acquire a controlling interest become “control shares” to which the voting restrictions described above apply.
47
DESCRIPTION OF PREFERRED STOCK
Shares of our Preferred Stock may be issued in one or more series, and our Board is authorized to determine the designation and to fix the number of shares of each series. Our Board is further authorized to fix and determine the dividend rate, premium or redemption rates, conversion rights, voting rights, preferences, privileges, restrictions, and other variations granted to or imposed upon any wholly unissued series of our Preferred Stock.
Prior to the issuance of shares of a series of Preferred Stock, our Board will adopt resolutions and file a certificate of designation with the Secretary of State of the State of Nevada. The certificate of designation will fix for each series the designation and number of shares and the rights, preferences, privileges, and restrictions of the shares including, but not limited to, the following:
|
• |
the voting rights, if any, of the Preferred Stock; |
|
• |
any rights and terms of redemption; |
|
• |
the dividend rate(s), period(s), and/or payment date(s) or method(s) of calculation applicable to the Preferred Stock; |
|
• |
whether dividends are cumulative or non-cumulative and, if cumulative, the date from which dividends on the Preferred Stock will accumulate; |
|
• |
the relative ranking and preferences of the preferred stock as to dividend rights and rights upon the liquidation, dissolution, or winding up of our affairs; |
|
• |
the terms and conditions, if applicable, upon which the Preferred Stock will be convertible into Common Stock, another series of Preferred Stock, or any other class of securities, including the conversion price (or manner of calculation) and conversion period; |
|
• |
the provision for redemption, if applicable, of the Preferred Stock; |
|
• |
the provisions for a sinking fund, if any, for the Preferred Stock; |
|
• |
the liquidation preferences, if any, for the Preferred Stock; |
|
• |
any limitations on the issuance of any class or series of Preferred Stock ranking senior to or on a parity with the class or series of Preferred Stock as to dividend rights and rights upon liquidation, dissolution, or winding up of our affairs; and |
|
• |
any other specific terms, preferences, rights, limitations, or restrictions of the Preferred Stock. |
In addition to the terms listed above, we will set forth in a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, the following terms relating to the series of Preferred Stock being offered:
|
• |
the number of shares of the preferred stock offered, the liquidation preference per share, the conversion rights, and the offering price of the Preferred Stock; |
|
• |
the procedures for any auction and remarketing, if any, for the Preferred Stock; |
|
• |
any listing of the Preferred Stock on any securities exchange; and |
|
• |
a discussion of any material and/or special United States federal income tax considerations applicable to the Preferred Stock. |
48
DESCRIPTION OF DEBT SECURITIES
The following description, together with the additional information we include in any applicable prospectus supplements or any related free writing prospectus or other offering materials, as applicable, summarizes the material terms and provisions of the debt securities that we may offer under this prospectus. While the terms we have summarized below will apply generally to any future debt securities we may offer pursuant to this prospectus, we will describe the particular terms of any debt securities that we may offer in more detail in the applicable prospectus supplement. If we so indicate in a prospectus supplement, the terms of any debt securities offered under such prospectus supplement may differ from the terms we describe below, and to the extent the terms set forth in a prospectus supplement differ from the terms described below, the terms set forth in the prospectus supplement or any related free writing prospectus or other offering materials, as applicable, shall control.
We may sell from time to time, in one or more offerings under this prospectus, debt securities, in one or more series. These debt securities that we may issue include senior debt securities, senior subordinated debt securities, subordinated debt securities, convertible debt securities, and exchangeable debt securities. We will issue any such senior debt securities under a senior indenture that we will enter into with a trustee to be named in the senior indenture. We will issue any such subordinated debt securities under a subordinated indenture, which we will enter into with a trustee to be named in the subordinated indenture. We use the term “indentures” to refer to either the senior indenture or the subordinated indenture, as applicable. The indentures will be qualified under the Trust Indenture Act of 1939, as amended (the “Trust Indenture Act”), as in effect on the date of the indenture. We use the term “debenture trustee” to refer to either the trustee under the senior indenture or the trustee under the subordinated indenture, as applicable.
The following summary description, together with the additional information we may include in any applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, does not purport to be complete and is subject to, and qualified in its entirety by reference to, the form of indenture filed as an exhibit to the Registration Statement of which the prospectus is a part, as it may be supplemented, amended, or modified from time to time, as well as the notes and supplemental agreement relating to each series of debt securities that will be incorporated by reference as exhibits to the Registration Statement that includes the prospectus or as exhibits to a Current Report on Form 8-K if we offer debt securities.
General
The indenture does not limit the amount of debt securities that may be issued thereunder, and each indenture provides that the specific terms of any series of debt securities shall be set forth in, or determined pursuant to, an authorizing resolution and/or a supplemental indenture, if any, relating to such series.
We may issue the debt securities issued under the indentures as “discount securities,” which means they may be sold at a discount below their stated principal amount. These debt securities, as well as other debt securities that are not issued at a discount, may be issued with “original issue discount,” or “OID,” for U.S. federal income tax purposes because of interest payment and other characteristics or terms of the debt securities. Material U.S. federal income tax considerations applicable to debt securities issued with OID will be described in more detail in any applicable prospectus supplement.
We will describe in the applicable prospectus supplement, the related free writing prospectus, or other offering materials, as applicable, the terms of the series of debt securities being offered, including:
|
|
• |
the title or designation; |
|
|
• |
the aggregate principal amount and any limit on the aggregate principal amount that may be issued; |
|
|
• |
the maturity date or dates on which principal will be payable; |
|
|
• |
the form of the debt securities of the series; |
49
|
|
• |
the applicability of any guarantees; |
|
|
• |
whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; |
|
|
• |
whether the debt securities rank as senior debt, senior subordinated debt, subordinated debt, or any combination thereof, and the terms of any subordination; |
|
|
• |
if the price (expressed as a percentage of the aggregate principal amount thereof) at which such debt securities will be issued is a price other than the principal amount thereof, the portion of the principal amount thereof payable upon declaration of acceleration of the maturity thereof, or, if applicable, the portion of the principal amount of such debt securities that is convertible into another security or the method by which any such portion shall be determined; |
|
|
• |
the interest rate or rates, which may be fixed or variable, or the method for determining the rate and the date interest will begin to accrue, the dates interest will be payable, and the regular record dates for interest payment dates or the method for determining such dates; |
|
|
• |
our right, if any, to defer payment of interest and the maximum length of any such deferral period; |
|
|
• |
if applicable, the date or dates after which, or the period or periods during which, and the price or prices at which, we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions and the terms of those redemption provisions; |
|
|
• |
the date or dates, if any, on which, and the price or prices at which we are obligated, pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option to purchase, the series of debt securities and the currency or currency unit in which the debt securities are payable; |
|
|
• |
the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral multiple thereof; |
|
|
• |
the place or places where payments will be payable; |
|
|
• |
whether the debt securities of that series shall be issued in whole or in part in the form of a global security or securities, the terms and conditions, if any, upon which such global security or securities may be exchanged in whole or in part for other individual securities; and the depositary for such global security or securities; |
|
|
• |
whether the indenture will restrict our ability to pay dividends or will require us to maintain any asset ratios or reserves; |
|
|
• |
if, other than the full principal amount thereof, the portion of the principal amount of debt securities of the series that shall be payable upon declaration of acceleration of the maturity thereof; |
|
|
• |
whether we will be restricted from incurring any additional indebtedness; |
|
|
• |
additions to or changes in the events of default with respect to the securities and any change in the right of the trustee or the holders to declare the principal, premium, if any, and interest, if any, with respect to such securities to be due and payable; |
|
|
|
|
|
• |
additions to or changes in the provisions relating to satisfaction and discharge of the indenture; |
|
|
• |
additions to or changes in the provisions relating to the modification of the indenture both with and without the consent of holders of debt securities issued under the indenture; |
50
|
|
• |
whether interest will be payable in cash or additional debt securities at our or the holders’ option and the terms and conditions upon which the election may be made; |
|
|
• |
the terms and conditions, if any, upon which we will pay amounts in addition to the stated interest, premium, if any, and principal amounts of the debt securities of the series to any holder that is not a “United States person” for federal tax purposes; |
|
|
• |
any restrictions on transfer, sale, or assignment of the debt securities of the series; |
|
|
• |
a discussion on any material or special U.S. federal income tax considerations applicable to a series of debt securities; and |
|
|
• |
any other specific terms, preferences, rights, or limitations of, or restrictions on, the debt securities, any other additions or changes in the provisions of the indenture, and any terms that may be required by us or advisable under applicable laws or regulations. |
We may issue debt securities that provide for an amount less than their stated principal amount to be due and payable upon declaration of acceleration of their maturity pursuant to the terms of the indenture. We will provide you with information on the federal income tax considerations and other special consideration applicable to any of these debt securities in the applicable prospectus supplement, related free writing prospectus, or other offering materials, as applicable.
Conversion or Exchange Rights
We will set forth in the applicable prospectus supplement, related free writing prospectus, or other offering materials, as applicable, the terms on which a series of debt securities may be convertible into or exchangeable for shares of our Common Stock, shares of our Preferred Stock, or other securities. We will include provisions as to settlement upon conversion or exchange and whether conversion or exchange is mandatory, at the option of the holder, or at our option. We may include provisions pursuant to which the number of shares of our Common Stock, shares of our Preferred Stock, or our other securities that the holders of the series of debt securities receive would be subject to adjustment.
Consolidation, Merger, or Sale; No Protection in Event of a Change of Control or Highly Leveraged Transaction
Unless we provide otherwise in the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, applicable to a particular series of debt securities, the indenture will contain covenant that restricts our ability to merge or consolidate, or sell, convey, transfer, or otherwise dispose of our assets as an entirety or substantially as an entirety, unless we are the surviving corporation or the successor to or acquirer of such assets (other than a subsidiary of ours) expressly assumes all of our obligations under the indenture or the debt securities, as appropriate. In addition, we cannot complete such a transaction unless immediately after completing the transaction, no event of default under the indenture, and no event that, after notice or lapse of time or both, would become an event of default under the indenture, has occurred and is continuing.
Unless we provide otherwise in the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable to a particular series of debt securities, the debt securities will not contain any provisions that may afford holders of the debt securities protection in the event we have a change of control or in the event of a highly leveraged transaction (whether or not such transaction results in a change of control), which could adversely affect holders of debt securities.
51
Events of Default Under the Indentures
Unless we provide otherwise in the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable to a particular series of debt securities, the following are events of default under the indentures with respect to any series of debt securities that we may issue:
|
|
• |
if we fail to pay interest when due and our failure continues for a period of 90 days; provided, however, that a valid extension of an interest payment period by us in accordance with the terms of any indenture supplement thereto shall not constitute a default in the payment of interest for this purpose; |
|
|
• |
if we fail to pay the principal of, or premium, if any, on any series of debt securities as and when the same shall become due and payable whether at maturity, upon redemption, by declaration or otherwise, or in any payment required by any sinking or analogous fund established with respect to such series; provided, however, that a valid extension of the maturity of such debt securities in accordance with the terms of any indenture supplement thereto shall not constitute a default in the payment of principal or premium, if any; |
|
|
• |
if we fail to observe or perform any other covenant or agreement contained in the debt securities or the indenture, other than a covenant specifically relating to another series of debt securities, and our failure continues for 90 days after we receive written notice of such failure, requiring the same to be remedied and stating that such is a notice of default thereunder, from the trustee or holders of at least 25% in aggregate principal amount of the outstanding debt securities of the applicable series; and |
|
|
• |
if specified events of bankruptcy, insolvency, or reorganization occur as to us. |
No event of default with respect to a particular series of debt securities (except as to certain events of bankruptcy, insolvency, or reorganization) necessarily constitutes an event of default with respect to any other series of debt securities. The occurrence of an event of default may constitute an event of default under any bank credit agreements we may have in existence from time to time. In addition, the occurrence of certain events of default or acceleration under the indenture may constitute an event of default under certain of our other indebtedness outstanding from time to time.
If an event of default with respect to debt securities of any series at the time outstanding occurs and is continuing, then the trustee or the holders of at least 25% in principal amount of the outstanding debt securities of that series may, by a notice in writing to us (and to the debenture trustee if given by the holders), declare to be due and payable immediately the principal (or, if the debt securities of that series are discount securities, that portion of the principal amount as may be specified in the terms of that series) of and premium and accrued and unpaid interest, if any, on all debt securities of that series. Before a judgment or decree for payment of the money due has been obtained with respect to debt securities of any series, the holders of a majority in principal amount of the outstanding debt securities of that series (or, at a meeting of holders of such series at which a quorum is present, the holders of a majority in principal amount of the debt securities of such series represented at such meeting) may rescind and annul the acceleration if all events of default, other than the non-payment of accelerated principal, premium, if any, and interest, if any, with respect to debt securities of that series, have been cured or waived as provided in the applicable indenture (including payments or deposits in respect of principal, premium or interest that had become due other than as a result of such acceleration). We refer you to the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, relating to any series of debt securities that are discount securities for the particular provisions relating to acceleration of a portion of the principal amount of such discount securities upon the occurrence of an event of default.
52
Subject to the terms of the indentures, if an event of default under an indenture shall occur and be continuing, the debenture trustee will be under no obligation to exercise any of its rights or powers under such indenture at the request or direction of any of the holders of the applicable series of debt securities, unless such holders have offered the debenture trustee reasonable indemnity. The holders of a majority in principal amount of the outstanding debt securities of any series will have the right to direct the time, method, and place of conducting any proceeding for any remedy available to the debenture trustee, or exercising any trust or power conferred on the debenture trustee, with respect to the debt securities of that series, provided, that:
|
|
• |
the direction so given by the holder is not in conflict with any law or the applicable indenture; and |
|
|
• |
subject to its duties under the Trust Indenture Act, the debenture trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved in the proceeding. |
A holder of the debt securities of any series will only have the right to institute a proceeding under the indentures or to appoint a receiver or trustee, or to seek other remedies if:
|
|
• |
the holder previously has given written notice to the debenture trustee of a continuing event of default with respect to that series; |
|
|
• |
the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request, and such holders have offered reasonable indemnity to the debenture trustee to institute the proceeding as trustee; and |
|
|
• |
the debenture trustee does not institute the proceeding, and does not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series (or at a meeting of holders of such series at which a quorum is present, the holders of a majority in principal amount of the debt securities of such series represented at such meeting) other conflicting directions within 60 days after the notice, request, and offer. |
These limitations do not apply to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the debt securities.
We will periodically file statements with the applicable debenture trustee regarding our compliance with specified covenants in the applicable indenture.
Modification of Indentures; Waiver
We and the debenture trustee may change the applicable indenture without the consent of any holders with respect to specific matters, including:
|
|
• |
to evidence the succession of another corporation to us and the assumption by any such successor of our covenants in such indenture and in the debt securities issued thereunder; |
|
|
• |
to add to our covenants or to surrender any right or power conferred on us pursuant to the indenture; |
|
|
• |
to establish the form and terms of debt securities issued thereunder; |
|
|
• |
to evidence and provide for a successor trustee under such indenture with respect to one or more series of debt securities issued thereunder or to provide for or facilitate the administration of the trusts under such indenture by more than one trustee; |
|
|
• |
to cure any ambiguity, to correct or supplement any provision in the indenture that may be defective or inconsistent with any other provision of the indenture or to make any other provisions with respect to matters or questions arising under such indenture; provided that no such action adversely affects the interests of the holders of any series of debt securities issued thereunder in any material respect; |
53
|
|
• |
to add to, delete from, or revise the conditions, limitations, and restrictions on the authorized amount, terms, or purposes of issue, authentication, and delivery of securities under the indenture; |
|
|
• |
to add any additional events of default with respect to all or any series of debt securities; |
|
|
• |
to supplement any of the provisions of the indenture as may be necessary to permit or facilitate the defeasance and discharge of any series of debt securities, provided that such action does not adversely affect the interests of any holder of an outstanding debt security of such series or any other security in any material respect; |
|
|
• |
to make provisions with respect to the conversion or exchange rights of holders of debt securities of any series; |
|
|
• |
to pledge to the trustee as security for the debt securities of any series any property or assets; |
|
|
• |
to add guarantees in respect of the debt securities of one or more series; |
|
|
• |
to change or eliminate any of the provisions of the indenture, provided that any such change or elimination becomes effective only when there is no security of any series outstanding created prior to the execution of such supplemental indenture that is entitled to the benefit of such provision; |
|
|
• |
to provide for certificated securities in addition to or in place of global securities; |
|
|
• |
to qualify such indenture under the Trust Indenture Act; |
|
|
• |
with respect to the debt securities of any series, to conform the text of the indenture or the debt securities of such series to any provision of the description thereof in our offering memorandum or prospectus relating to the initial offering of such debt securities, to the extent that such provision, in our good faith judgment, was intended to be a verbatim recitation of a provision of the indenture or such securities; or |
|
|
• |
to make any other change that does not adversely affect the rights of holders of any series of debt securities issued thereunder in any material respect. |
In addition, under the indentures, the rights of holders of a series of debt securities may be changed by us and the debenture trustee with the written consent of the holders of at least a majority in aggregate principal amount of the outstanding debt securities of each series (or, at a meeting of holders of such series at which a quorum is present, the holders of a majority in principal amount of the debt securities of such series represented at such meeting) that is affected. However, the debenture trustee and we may make the following changes only with the consent of each holder of any outstanding debt security affected:
|
|
• |
extending the fixed maturity of the series of debt securities; |
|
|
• |
reducing the principal amount, reducing the rate of, or extending the time of payment of interest, or any premium payable upon the redemption of any debt securities; |
|
|
• |
reducing the principal amount of discount securities payable upon acceleration of maturity; |
|
|
• |
making the principal of or premium or interest on any debt security payable in currency other than that stated in the debt security; |
|
|
• |
impair the right to institute suit for the enforcement of any payment on any debt security when due; |
|
|
• |
if applicable, adversely affect the right of a holder to confer or exchange a debt security; or |
|
|
• |
reducing the percentage of debt securities, the holders of which are required to consent to any amendment or waiver. |
54
Except for certain specified provisions, the holders of at least a majority in principal amount of the outstanding debt securities of any series (or, at a meeting of holders of such series at which a quorum is present, the holders of a majority in principal amount of the debt securities of such series represented at such meeting) may on behalf of the holders of all debt securities of that series waive our compliance with provisions of the indenture. The holders of a majority in principal amount of the outstanding debt securities of any series may, on behalf of the holders of all the debt securities of such series, waive any past default under the indenture with respect to that series and its consequences, except a default in the payment of the principal of, premium, or any interest on any debt security of that series or in respect of a covenant or provision, which cannot be modified or amended without the consent of the holder of each outstanding debt security of the series affected; provided, however, that the holders of a majority in principal amount of the outstanding debt securities of any series may rescind an acceleration and its consequences, including any related payment default that resulted from the acceleration.
Discharge, Defeasance, and Covenant Defeasance
We can discharge or decrease our obligations under the indenture as stated below.
We may discharge obligations to holders of any series of debt securities that have not already been delivered to the trustee for cancellation and that have either become due and payable or are by their terms to become due and payable, or are scheduled for redemption, within one year. We may effect a discharge by irrevocably depositing with the trustee cash or government obligations, as trust funds, in an amount certified to be enough to pay, when due, whether at maturity, upon redemption or otherwise, the principal of, and any premium and interest on, the debt securities and any mandatory sinking fund payments.
Unless otherwise provided in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, we may also discharge any and all of our obligations to holders of any series of debt securities at any time, which we refer to as defeasance. We may also be released from the obligations imposed by any covenants of any outstanding series of debt securities and provisions of the indenture, and we may omit to comply with those covenants without creating an event of default under the trust declaration, which we refer to as covenant defeasance. We may effect defeasance and covenant defeasance only if, among other things:
|
|
• |
we irrevocably deposit with the trustee cash or government obligations denominated in the currency of the debt securities, as trust funds, in an amount certified to be enough to pay at maturity, or upon redemption, the principal (including any mandatory sinking fund payments) of, and any premium and interest on, all outstanding debt securities of the series; and |
|
|
• |
we deliver to the trustee an opinion of counsel from a nationally recognized law firm to the effect that the holders of the series of debt securities will not recognize income, gain or loss for U.S. federal income tax purposes as a result of the defeasance or covenant defeasance and that defeasance or covenant defeasance will not otherwise alter the holders’ U.S. federal income tax treatment of principal, and any premium and interest payments on, the series of debt securities. |
In the case of a defeasance by us, the opinion we deliver must be based on a ruling of the Internal Revenue Service issued, or a change in U.S. federal income tax law occurring, after the date of the indenture, since such a result would not occur under the U.S. federal income tax laws in effect on that date.
Although we may discharge or decrease our obligations under the indenture as described in the two preceding paragraphs, we may not avoid, among other things, our duty to register the transfer or exchange of any series of debt securities, to replace any temporary, mutilated, destroyed, lost, or stolen series of debt securities or to maintain an office or agency in respect of any series of debt securities.
55
Registered Global Securities and Book Entry System
The debt securities of a series may be issued in whole or in part in book-entry form and will be represented by one or more fully registered global securities. We will deposit any registered global securities with a depositary or with a nominee for a depositary identified in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials and registered in the name of such depositary or nominee. In such case, we will issue one or more registered global securities denominated in an amount equal to the aggregate principal amount of all of the debt securities of the series to be issued and represented by such registered global security or securities. This means that we will not issue certificates to each holder.
Unless and until it is exchanged in whole or in part for debt securities in definitive registered form, a registered global security may not be transferred except as a whole:
|
|
• |
by the depositary for the registered global security to its nominee; |
|
|
• |
by a nominee of the depositary to the depositary or another nominee of the depositary; or |
|
|
• |
by the depositary or its nominee to a successor of the depositary or a nominee of the successor. |
The prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, relating to a series of debt securities will describe the specific terms of the depositary arrangement involving any portion of the series represented by a registered global security. We anticipate that the following provisions will apply to all depositary arrangements for debt securities:
|
|
• |
ownership of beneficial interests in a registered global security will be limited to persons that have accounts with the depositary for such registered global security, these persons being referred to as “participants,” or persons that may hold interests through participants; |
|
|
• |
upon the issuance of a registered global security, the depositary for the registered global security will credit, on its book-entry registration and transfer system, the participants’ accounts with the respective principal amounts of the debt securities represented by the registered global security beneficially owned by the participants; |
|
|
• |
any dealers, underwriters, or agents participating in the distribution of the debt securities will designate the accounts to be credited; and |
|
|
• |
ownership of beneficial interest in the registered global security will be shown on, and the transfer of the ownership interest will be effected only through, records maintained by the depositary for the registered global security for interests of participants, and on the records of participants for interests of persons holding through participants. |
The laws of some states may require that specified purchasers of securities take physical delivery of the securities in definitive form. These laws may limit the ability of those persons to own, transfer, or pledge beneficial interests in registered global securities.
So long as the depositary for a registered global security, or its nominee, is the registered owner of the registered global security, the depositary or such nominee, as the case may be, will be considered the sole owner or holder of the debt securities represented by the registered global security for all purposes under the indenture. Except as stated below, owners of beneficial interests in a registered global security:
|
|
• |
will not be entitled to have the debt securities represented by a registered global security registered in their names; |
|
|
• |
will not receive or be entitled to receive physical delivery of the debt securities in the definitive form; and |
|
|
• |
will not be considered the owners or holders of the debt securities under the relevant indenture. |
56
|
|
Accordingly, each person owning a beneficial interest in a registered global security must rely on the procedures of the depositary for the registered global security and, if the person is not a participant, on the procedures of a participant through which the person owns its interest, to exercise any rights of a holder under the indenture.
We understand that, under existing industry practices, if we request any action of holders or if an owner of a beneficial interest in a registered global security desires to give or take any action that a holder is entitled to give or take under the indenture, the depositary for the registered global security would authorize the participants holding the relevant beneficial interests to give or take the action, and the participants would authorize beneficial owners owning through the participants to give or take the action or would otherwise act upon the instructions of beneficial owners holding through them.
We will make payments of principal and premium, if any, and interest, if any, on debt securities represented by a registered global security registered in the name of a depositary or its nominee to the depositary or its nominee, as the case may be, as the registered owners of the registered global security. Neither we nor the trustee, or any other agent of ours or the trustee will be responsible or liable for any aspect of the records relating to, or payments made on account of, beneficial ownership interests in the registered global security or for maintaining, supervising, or reviewing any records relating to the beneficial ownership interests.
We expect that the depositary for any debt securities represented by a registered global security, upon receipt of any payments of principal and premium, if any, and interest, if any, in respect of the registered global security, will immediately credit participants' accounts with payments in amounts proportionate to their respective beneficial interests in the registered global security as shown on the records of the depositary. We also expect that standing customer instructions and customary practices will govern payments by participants to owners of beneficial interests in the registered global security held through the participants, as is now the case with the securities held for the accounts of customers in bearer form or registered in “street name.” We also expect that any of these payments will be the responsibility of the participants.
If the depositary for any debt securities represented by a registered global security is at any time unwilling or unable to continue as depositary or stops being a clearing agency registered under the Exchange Act, we will appoint an eligible successor depositary. If we fail to appoint an eligible successor depositary within 90 days, we will issue the debt securities in definitive form in exchange for the registered global security. In addition, we may at any time and in our sole discretion decide not to have any of the debt securities of a series represented by one or more registered global securities. In that event, we will issue debt securities of the series in a definitive form in exchange for all of the registered global securities representing the debt securities. The trustee will register any debt securities issued in definitive form in exchange for a registered global security in the name or names as the depositary, based upon instructions from its participants, shall instruct the trustee.
Information Concerning the Debenture Trustee
The debenture trustee, other than during the occurrence and continuance of an event of default under the applicable indenture, undertakes to perform only those duties as are specifically set forth in the applicable indenture. Upon an event of default under an indenture, the debenture trustee under such indenture must use the same degree of care as a prudent person would exercise or use in the conduct of his or her own affairs. Subject to this provision, the debenture trustee is under no obligation to exercise any of the powers given it by the indentures at the request of any holder of debt securities unless it is offered reasonable security and indemnity against the costs, expenses, and liabilities that it might incur.
Payment and Paying Agents
Unless we other indicate in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, we will make payment of the interest on any debt securities on any interest payment date to the person in whose name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular record date for the interest.
We will pay principal of and any premium and interest on the debt securities of a particular series at the office of the paying agents designated by us, except that unless we otherwise indicate in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, we will make interest payments by check which we will mail to the holder. Unless we otherwise indicate in a prospectus
57
supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, we will designate the corporate trust office of the debenture trustee as our sole paying agent for payments with respect to debt securities of each series. We will name in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, any other paying agents that we initially designate for the debt securities of a particular series. We will maintain a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying agent or the debenture trustee for the payment of the principal of or any premium or interest on any debt securities which remains unclaimed at the end of two years after such principal, premium, or interest has become due and payable will be repaid to us, and the holder of the security thereafter may look only to us for payment thereof.
Governing Law
The indentures and the debt securities will be governed by and construed in accordance with the laws of the State of New York, except to the extent that the Trust Indenture Act is applicable.
Subordination of Subordinated Debt Securities
Our obligations pursuant to any subordinated debt securities will be unsecured and will be subordinate and junior in priority of payment to certain of our other indebtedness to the extent described in a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable.
Outstanding Debt Securities
As of December 15, 2020, we had the following debt securities outstanding:
|
|
• |
Secured Revolving Line of Credit Promissory Note, dated August 28, 2019, issued to Isaac Capital Group LLC in the original principal amount of $2,500,000. The promissory note matures on December 31, 2020 and bears interest at 8.75% per annum. |
58
General
We may issue warrants to purchase debt securities, shares of our Common Stock, shares of our Preferred Stock, or any combination of these securities. We may issue the warrants independently or together with any underlying securities, and the warrants may be attached or separate from the underlying securities. We may also issue a series of warrants under a separate warrant agreement to be entered into between a warrant agent and us. The warrant agent will act solely as our agent in connection with the warrants of such series and will not assume any obligation or relationship of agency for or with holders or beneficial owners of warrants.
The following description is a summary of selected provisions relating to the warrants that we may issue. The summary is not complete. When warrants are offered in the future, a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, will explain the particular terms of those securities and the extent to which these general provisions may apply. The specific terms of the warrants as described in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials will supplement and, if applicable, may modify or replace the general terms described in this section.
This summary and any description of warrants in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials is subject to and is qualified in its entirety by reference to all the provisions of any specific warrant document or agreement, which we will file with the SEC for incorporation by reference into this prospectus. See “Available Information” and “Incorporation of Certain Information by Reference” for information on how to obtain a copy of a warrant document when it is filed.
When we refer to a series of warrants, we mean all warrants issued as part of the same series under the applicable warrant agreement.
Terms
The applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials may describe the terms of any warrants that we may offer, including but not limited to the following:
|
• |
the title of the warrants; |
|
• |
the total number of warrants; |
|
• |
the price or prices at which the warrants will be issued; |
|
• |
the currency or currencies that investors may use to pay for the warrants; |
|
• |
the date on which the right to exercise the warrants will commence and the date on which the right will expire; |
|
• |
whether the warrants will be issued in registered form or bearer form; |
|
• |
information with respect to book-entry procedures, if any; |
|
• |
if applicable, the minimum or maximum amount of warrants that may be exercised at any one time; |
|
• |
if applicable, the designation and terms of the underlying securities with which the warrants are issued and the number of warrants issued with each underlying security; |
59
|
• |
if applicable, the date on and after which the warrants and the related underlying securities will be separately transferable; |
|
• |
if applicable, a discussion of material United States federal income tax considerations; |
|
• |
if applicable, the terms of redemption of the warrants; |
|
• |
the identity of the warrant agent, if any; |
|
• |
the procedures and conditions relating to the exercise of the warrants; and |
|
• |
any other terms of the warrants, including terms, procedures, and limitations relating to the exchange and exercise of the warrants. |
Warrant Agreements
We may issue the warrants in one or more series under one or more warrant agreements, each to be entered into between a bank, trust company, or other financial institution as warrant agent, and us. We may add, replace, or terminate warrant agents from time to time. We may also choose to act as our own warrant agent or may choose one of our subsidiaries to do so.
The warrant agent under a warrant agreement will act solely as our agent in connection with the warrants issued under that agreement. The warrant agent will not assume any obligation or relationship of agency or trust for or with any holders of those warrants. Any holder of warrants may, without the consent of any other person, enforce by appropriate legal action, on its own behalf, its right to exercise those warrants in accordance with their terms. Until the warrant is properly exercised, no holder of any warrant will be entitled to any rights of a holder of the warrant property purchasable upon exercise of the warrant.
Form, Exchange, and Transfer
We may issue the warrants in registered form or bearer form. Warrants issued in registered form, i.e., book-entry form, will be represented by a global security registered in the name of a depository, which will be the holder of all the warrants represented by the global security. Those investors who own beneficial interests in a global warrant will do so through participants in the depository’s system, and the rights of these indirect owners will be governed solely by the applicable procedures of the depository and its participants. In addition, we may issue warrants in non-global form, i.e., bearer form. If any warrants are issued in non-global form, warrant certificates may be exchanged for new warrant certificates of different denominations, and holders may exchange, transfer, or exercise their warrants at the warrant agent’s office or any other office indicated in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials.
Prior to the exercise of their warrants, holders of warrants exercisable for debt securities will not have any of the rights of holders of the debt securities purchasable upon such exercise and will not be entitled to payments of principal (or premium, if any) or interest, if any, on the debt securities purchasable upon such exercise. Prior to the exercise of their warrants, holders of warrants exercisable for shares of Common Stock or shares of Preferred Stock will not have any rights of holders of the shares of Common Stock or the shares of Preferred Stock purchasable upon such exercise and will not be entitled to dividend payments, if any, or voting rights of the shares of Common Stock or the shares of Preferred Stock purchasable upon such exercise.
60
Exercise of Warrants
A warrant will entitle the holder to purchase for cash an amount of securities at an exercise price that will be stated in, or that will be determinable as described in, the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials. Warrants may be exercised at any time from the initial exercise date and time through and including the close of business on the expiration date set forth in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials. After the close of business on the expiration date, unexercised warrants will become void. Warrants may be redeemed as set forth in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials.
Warrants may be exercised as set forth in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials. Upon receipt of payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, we will forward, as soon as practicable, the securities purchasable upon such exercise. If less than all of the warrants represented by such warrant certificate are exercised, a new warrant certificate will be issued for the remaining warrants.
61
We may issue rights to purchase our debt securities, shares of our Common Stock, or shares of our Preferred Stock. These rights may be issued independently or together with any other security offered hereby and may or may not be transferable by the stockholder receiving the rights in such offering. In connection with any offering of such rights, we may enter into a standby arrangement with one or more underwriters or other purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for after such offering.
Each series of rights will be issued under a separate rights agreement that we will enter with a bank or trust company, as rights agent, all of which will be set forth in the relevant offering material. The rights agent will act solely as our agent in connection with the certificates relating to the rights and will not assume any obligation or relationship of agency or trust with any holders of rights certificates or beneficial owners of rights.
The following description is a summary of selected provisions relating to rights that we may offer. The summary is not complete. When rights are offered in the future, a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, will explain the particular terms of those securities and the extent to which these general provisions may apply. The specific terms of the rights as described in a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, will supplement and, if applicable, may modify or replace the general terms described in this section.
This summary and any description of rights in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials is subject to and is qualified in its entirety by reference to the rights agreement and the rights certificates. We will file each of these documents, as applicable, with the SEC and incorporate them by reference as an exhibit to the Registration Statement of which this prospectus is a part on or before the time we issue a series of rights. See “Available Information” and “Incorporation of Certain Documents by Reference” above for information on how to obtain a copy of a document when it is filed.
The applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials may describe:
|
• |
in the case of a distribution of rights to our stockholders, the date of determining the stockholders entitled to the rights distribution; |
|
• |
in the case of a distribution of rights to our stockholders, the number of rights issued or to be issued to each stockholder; |
|
• |
the exercise price payable for the underlying debt securities, shares of our Common Stock or shares of our Preferred Stock upon the exercise of the rights; |
|
• |
the number and terms of the underlying debt securities, shares of our Common Stock or shares of our Preferred Stock that may be purchased per each right; |
|
• |
the extent to which the rights are transferable; |
|
• |
the date on which the holder’s ability to exercise the rights shall commence, and the date on which the rights shall expire; |
|
• |
the extent to which the rights may include an over-subscription privilege with respect to unsubscribed securities; |
|
• |
if applicable, the material terms of any standby underwriting or purchase arrangement entered into by us in connection with the offering of such rights; and |
|
• |
any other terms of the rights, including, but not limited to, the terms, procedures, conditions, and limitations relating to the exchange and exercise of the rights. |
The provisions described in this section, as well as those described under “—Description of Debt Securities” and “—Description of Capital Stock” above, will apply, as applicable, to any rights we offer.
62
General
We may issue units composed of (i) our debt securities, (ii) shares of our Common Stock, (iii) shares of our Preferred Stock, (iv) warrants to purchase our debt securities, shares of our Common Stock, or shares of our Preferred Stock or any combination of these securities, and (v) rights to purchase our debt securities, shares of our Common Stock, or shares of our Preferred Stock in any combination. We will issue each unit so that the holder of the unit is also the holder of each security included in the unit. As a result, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
The following description is a summary of selected provisions relating to units that we may offer. The summary is not complete. When units are offered in the future, a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, will explain the particular terms of those securities and the extent to which these general provisions may apply. The specific terms of the units as described in a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, will supplement and, if applicable, may modify or replace the general terms described in this section.
This summary and any description of units in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials is subject to and is qualified in its entirety by reference to the unit agreement, collateral arrangements and depositary arrangements, if applicable. We will file these documents with the SEC for incorporation by reference into this prospectus, as applicable. See “Available Information” and “Incorporation of Certain Information by Reference” for information on how to obtain a copy of a document when it is filed.
The applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials may describe:
|
• |
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
|
• |
any provisions for the issuance, payment, settlement, transfer, or exchange of the units or of the securities composing the units; |
|
• |
whether the units will be issued in fully registered or global form; and |
|
• |
any other terms of the units. |
The applicable provisions described in this section, as well as those described under “Description of Debt Securities,” “Description of Capital Stock” and “Description of Warrants,” will apply to each unit and to each security included in each unit, respectively.
Unless otherwise indicated in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, we intend to use the net proceeds from the sale of securities for general corporate purposes.
63
We may sell the securities through underwriters or dealers, through agents, directly to one or more purchasers, through a rights offering, or otherwise. We will describe the terms of the offering of the securities in a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, including:
|
• |
the name or names of any underwriters, if any; |
|
• |
the purchase price of the securities and the proceeds we will receive from the sale; |
|
• |
any underwriting discounts and other items constituting underwriters’ compensation; |
|
• |
any initial public offering price; |
|
• |
any discounts or concessions allowed or reallowed or paid to dealers; and |
|
• |
any securities exchange or market on which the securities may be listed. |
Only underwriters we name in the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, are underwriters of the securities offered thereby.
The distribution of securities may be effected, from time to time, in one or more transactions, including:
|
• |
block transactions (which may involve crosses) and transactions on The Nasdaq Capital Market or any other organized market on which the securities may be traded; |
|
• |
purchases by a broker-dealer as principal and resale by the broker-dealer for its own account pursuant to a prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable; |
|
• |
ordinary brokerage transactions and transactions in which a broker-dealer solicits purchasers; |
|
• |
sales “at the market” to or through a market maker or into an existing trading market, on an exchange or otherwise; and |
|
• |
sales in other ways not involving market makers or established trading markets, including direct sales to purchasers. |
The securities may be sold at a fixed price or prices, which may be changed, or at market prices prevailing at the time of sale, at prices relating to the prevailing market prices, or at negotiated prices. The consideration may be cash or another form negotiated by the parties. Agents, underwriters, or broker-dealers may be paid compensation for offering and selling the securities. That compensation may be in the form of discounts, concessions, or commissions to be received from us or from the purchasers of the securities. Dealers and agents participating in the distribution of the securities may be deemed to be underwriters and compensation received by them on resale of the securities may be deemed to be underwriting discounts and commissions under the Securities Act. If such dealers or agents were deemed to be underwriters, they may be subject to statutory liabilities under the Securities Act.
We may also make direct sales through subscription rights distributed to our existing stockholders on a pro rata basis, which may or may not be transferable. In any distribution of subscription rights to our stockholders, if all of the underlying securities are not subscribed for, we may then sell the unsubscribed securities directly to third parties or may engage the services of one or more underwriters, dealers, or agents, including standby underwriters, to sell the unsubscribed securities to third parties.
64
Some or all of the securities that we offer though this prospectus may be new issues of securities with no established trading market. Any underwriters to whom we sell our securities for public offering and sale may make a market in those securities, but they will not be obligated to do so and they may discontinue any market making at any time without notice. Accordingly, we cannot assure you of the liquidity of, or continued trading markets for, any securities that we offer.
Agents may, from time to time, solicit offers to purchase the securities. If required, we will name in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus or other offering materials, as applicable, any agent involved in the offer or sale of the securities and set forth any compensation payable to the agent. Unless otherwise indicated, any agent will be acting on a best efforts basis for the period of its appointment. Any agent selling the securities covered by this prospectus may be deemed to be an underwriter, as that term is defined in the Securities Act, of the securities.
If underwriters are used in an offering, securities will be acquired by the underwriters for their own account and may be resold, from time to time, in one or more transactions, including negotiated transactions, at a fixed public offering price, or at varying prices determined at the time of sale, or under delayed delivery contracts or other contractual commitments. Securities may be offered to the public either through underwriting syndicates represented by one or more managing underwriters or directly by one or more firms acting as underwriters. If an underwriter or underwriters are used in the sale of securities, an underwriting agreement will be executed with the underwriter or underwriters at the time an agreement for the sale is reached. The applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials will set forth the managing underwriter or underwriters, as well as any other underwriter or underwriters, with respect to a particular underwritten offering of securities, and will set forth the terms of the transactions, including compensation of the underwriters and dealers and the public offering price, if applicable. The prospectus, and the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials will be used by the underwriters to resell the securities.
If a dealer is used in the sale of the securities, we or an underwriter will sell the securities to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. To the extent required, we will set forth in the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, the name of the dealer and the terms of the transactions.
We may directly solicit offers to purchase the securities and may make sales of securities directly to institutional investors or others. These persons may be deemed to be underwriters within the meaning of the Securities Act with respect to any resale of the securities. To the extent required, the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, will describe the terms of any such sales, including the terms of any bidding or auction process, if used.
Agents, underwriters, and dealers may be entitled under agreements that may be entered into with us to indemnification against specified liabilities, including liabilities incurred under the Securities Act, or to contribution to payments they may be required to make in respect of such liabilities. If required, the prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials, as applicable, will describe the terms and conditions of such indemnification or contribution. Some of the agents, underwriters or dealers, or their affiliates may be customers of, engage in transactions with or perform services for us, our subsidiaries or affiliates in the ordinary course of business.
Under the securities laws of some states, the securities offered by this prospectus may be sold in those states only through registered or licensed brokers or dealers.
65
Any person participating in the distribution of Common Stock registered under the Registration Statement that includes this prospectus will be subject to applicable provisions of the Exchange Act, and the applicable SEC rules and regulations, including, among others, Regulation M, which may limit the timing of purchases and sales of any of our Common Stock by any such person. Furthermore, Regulation M may restrict the ability of any person engaged in the distribution of our Common Stock to engage in market-making activities with respect to our Common Stock. These restrictions may affect the marketability of our Common Stock and the ability of any person or entity to engage in market-making activities with respect to our Common Stock.
Certain persons participating in an offering may engage in over-allotment, stabilizing transactions, short-covering transactions, and penalty bids in accordance with Regulation M under the Exchange Act that stabilize, maintain, or otherwise affect the price of the offered securities. If any such activities will occur, they will be described in the applicable prospectus supplement, information or document incorporated by reference, related free writing prospectus, or other offering materials.
To the extent required, this prospectus may be amended or supplemented from time to time to describe a specific plan of distribution.
All securities we offer other than shares of Common Stock will be new issues of securities with no established trading market. Any underwriters may make a market in these securities but will not be obligated to do so and may discontinue any market making at any time without notice. We cannot guarantee the liquidity of the trading markets for any securities.
Unless otherwise indicated in the applicable prospectus supplement, Clark Hill PLC, Los Angeles, California, will provide opinions regarding the validity of any securities offered by this prospectus. Clark Hill PLC may also provide opinions regarding certain other matters. The legality of the securities for any underwriters, dealers, or agents will be passed upon by counsel as may be specified in the applicable prospectus supplement.
The consolidated financial statements incorporated in this Prospectus by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 28, 2019, have been audited by WSRP, LLC, an independent registered public accounting firm, as stated in their reports incorporated by reference herein, and have been so incorporated in reliance upon such reports and upon the authority of such firm as experts in accounting and auditing.
The consolidated financial statements of the Company at December 29, 2018 incorporated in this Prospectus by reference to the Registrant’s Annual Report on Form 10-K, as amended, for the year ended December 29, 2018, have been audited by SingerLewak LLP, an independent registered public accounting firm, as stated in their report incorporated by reference herein, and have been so incorporated in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
66
PART II. INFORMATION NOT REQUIRED IN THE PROSPECTUS
ITEM 14. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth all expenses to be paid by the Registrant in connection with this offering.
|
Fee |
|
|
Total |
|
|
SEC registration fee |
|
$ |
12,980 |
|
|
FINRA filing fee |
|
|
* |
|
|
Nasdaq listing fee |
|
|
* |
|
|
Printing |
|
|
* |
|
|
Legal fees and expenses |
|
|
* |
|
|
Accounting fees and expenses |
|
|
* |
|
|
Transfer agent and registrar fees |
|
|
* |
|
|
Miscellaneous |
|
|
* |
|
|
Total |
|
$ |
* |
|
* Fees and expenses (other than the SEC registration fee to be paid upon filing of this registration statement) will depend on the securities offered, the number of issuances and the nature of the offerings, and cannot be estimated at this time.
ITEM 15. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Nevada Revised Statutes (“NRS”) 78.138(7) provides that, subject to limited statutory exceptions and unless the Articles of Incorporation or an amendment thereto (in each case filed on or after October 1, 2003) provide for greater individual liability, a director or officer is not individually liable to a corporation or its stockholders or creditors for any damages as a result of any act or failure to act in his or her capacity as a director or officer unless determined that the presumption that directors and officers are presumed to act in good faith, on an informed basis and with a view to the interest of the corporation has been rebutted and it is proven that: (i) the act or failure to act constituted a breach of his or her fiduciary duties as a director or officer and (ii) the breach of those duties involved intentional misconduct, fraud, or a knowing violation of law.
NRS 78.7502(1) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit, or proceeding, whether civil, criminal, administrative, or investigative (other than an action by or in the right of the corporation), by reason of the fact that the person is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise, against expenses, including attorneys’ fees, judgments, fines, and amounts paid in settlement actually and reasonably incurred by the person in connection with the action, suit, or proceeding if the person (i) is not liable pursuant to NRS 78.138 or (ii) acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe the conduct was unlawful. NRS 78.7502(2) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending, or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise against expenses, including amounts paid in settlement and attorneys’ fees actually and reasonably incurred by the person in connection with the defense or settlement of the action or suit if the person (a) is not liable pursuant to NRS 78.138 or (b) acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation. Any discretionary indemnification pursuant to NRS 78.7502, unless ordered by a court or advanced pursuant to NRS 78.751(2), may be made by the corporation only as authorized in each specific case upon a determination that the indemnification of a director, officer, employee, or agent of a corporation is proper under the circumstances. Such determination must be made by (x) the stockholders, (y) the board of directors, by majority vote of a quorum consisting of directors who were not parties to the action, suit, or proceeding, or (z) independent legal counsel, in a written opinion, if (1) a majority vote of a quorum consisting of directors who were not parties to the action, suit, or proceeding so orders or (2) a quorum consisting of directors who were not parties to the action, suit, or proceeding cannot be obtained.
II-1
The termination of any action, suit, or proceeding by judgment, order, settlement, conviction, or upon a plea of nolo contendere or its equivalent, does not, of itself, create a presumption that the person is liable pursuant to NRS 78.138 or did not act in good faith and in a manner that he or she reasonably believed to be in or not opposed to the best interests of the corporation or that, with respect to any criminal action or proceeding, he or she had reasonable cause to believe that the conduct was unlawful.
NRS 78.7502(2)(b) provides that indemnification may not be made for any claim, issue, or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals therefrom, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
NRS 78.751(1), requiring mandatory indemnification of officers, directors, employees, and agents, provides that a corporation shall indemnify any person in such a role to the extent that the person is successful on the merits or otherwise in defense of: (a) any threatened, pending or completed action, suit, or proceeding, whether civil, criminal, administrative or investigative, including, without limitation, an action by or in the right of the corporation, by reason of the fact that the person is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise or (b) any claim, issue, or matter therein, against expenses actually and reasonably incurred by the person in connection with defending the action, including, without limitation, attorney’s fees.
NRS 78.751(2) provides that, unless otherwise restricted by the corporation’s articles of incorporation or bylaws, or an agreement made by the corporation, the corporation may pay expenses of officers and directors incurred in defending a civil or criminal action, suit, or proceeding as they are incurred and in advance of the final disposition of the action, suit, or proceeding, upon receipt of an undertaking by or on behalf of the director or officer to repay the amount if it is ultimately determined by a court of competent jurisdiction that the director or officer was not entitled to be indemnified by the corporation. The articles of incorporation, the bylaws, or an agreement made by the corporation may require the corporation to pay such expenses upon receipt of such an undertaking.
Under the NRS, the indemnification pursuant to NRS 78.7502 and advancement of expenses authorized in or ordered by a court pursuant to NRS 78.751:
|
|
• |
Does not exclude any other rights to which a person seeking indemnification or advancement of expenses may be entitled under the articles of incorporation or any bylaw, agreement, vote of stockholders or disinterested directors or otherwise, for either an action in the person’s official capacity or an action in another capacity while holding office, except that indemnification, unless ordered by a court pursuant to NRS 78.7502 or for the advancement of expenses made pursuant to NRS 78.751(2), may not be made to or on behalf of any director or officer finally adjudged by a court of competent jurisdiction, after exhaustion of any appeals taken therefrom, to be liable for intentional misconduct, fraud or a knowing violation of the law, and such misconduct, fraud or violation was material to the cause of action; and |
|
|
• |
Continues for a person who has ceased to be a director, officer, employee, or agent and inures to the benefit of the heirs, executors, and administrators of such a person. |
Unless the articles of incorporation, the bylaws, or an agreement made by a corporation provide otherwise, if a person is entitled to indemnification or the advancement of expenses from the corporation and any other person, the corporation is the primary obligor with respect to such indemnification or advancement. A right to indemnification or to advancement of expenses arising under a provision of the articles of incorporation or any bylaw is not eliminated or impaired by an amendment to such provision after the occurrence of the act or omission that is the subject of the civil, criminal, administrative or investigative action, suit, or proceeding for which indemnification or advancement of expenses is sought, unless the provision in effect at the time of such act or omission explicitly authorizes such elimination or impairment after such act or omission has occurred.
II-2
The Company’s Articles of Incorporation provide that, to the fullest extent permitted under the NRS (including, without limitation, to the fullest extent permitted under NRS 78.7502 and 78.751(3)) and other applicable law, the Company shall indemnify its directors and officers in their respective capacities as such. The Company’s Articles of Incorporation further provide that the liability of its directors and officers shall be eliminated or limited to the fullest extent permitted by the NRS.
The Registrant intends to maintain insurance on behalf of the Registrant and any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in that capacity, subject to certain exclusions and limits of the amount of coverage.
II-3
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
Exhibits
|
Exhibit No. |
|
Description |
|
1.1* |
|
Form of Underwriting Agreement with respect to Debt Securities |
|
1.2* |
|
Form of Underwriting Agreement with respect to Common Stock |
|
1.3* |
|
Form of Underwriting Agreement with respect to Preferred Stock |
|
1.4* |
|
Form of Underwriting Agreement with respect to Warrants |
|
1.5* |
|
Form of Underwriting Agreement with respect to Units |
|
3.1 |
|
Articles of Incorporation of Appliance Recycling Centers of America, Inc. (incorporated by reference to Exhibit 3.3 of the Company’s Current Report on Form 8-K filed with the SEC on March 13, 2018) |
|
3.2 |
|
Articles of Conversion (incorporated by reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K filed with the SEC on March 13, 2018) |
|
3.3 |
|
Articles of Conversion (incorporated by reference to Exhibit 3.2 of the Company’s Current Report on Form 8-K filed with the SEC on March 13, 2018) |
|
3.4 |
|
Certificate of Correction to Articles of Incorporation (incorporated by reference to Exhibit 3.1 of the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2018) |
|
3.5 |
|
Certificate of Change (incorporated by reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K filed with the SEC on April 22, 2019) |
|
3.6 |
|
(incorporated by reference to Exhibit 3.7 of the Company’s Current Report on Form 8-K filed with the SEC on June 24, 2019) |
|
3.7 |
|
Certificate of Designation of Powers, Preferences, and Rights of Series A-1 Convertible Preferred Stock of JanOne Inc. (formerly known as Appliance Recycling Centers of America, Inc.) (incorporated by reference to Exhibit 3.8 of the Company’s Current Report on Form 8-K filed with the SEC on June 24, 2019) |
|
3.8 |
|
Articles of Incorporation of JanOne Inc. (the Name Change Subsidiary), filed with the Secretary of State of the State of Nevada on September 6, 2019 (incorporated by reference to Exhibit 3.10 of the Company’s Current Report on Form 8-K filed with the SEC on June 24, 2019) |
|
3.9 |
|
Articles of Merger for JanOne Inc. into Appliance Recycling Centers of America, Inc., filed with the Secretary of the State of Nevada on September 9, 2019, and effective on September 10, 2019 (incorporated by reference to Exhibit 3.10 of the Company’s Current Report on Form 8-K filed with the SEC on June 24, 2019) |
|
3.10 |
|
Amended and Restated Certificate of Designation for the Preferences, Rights, and Limitations of the Series A-1 Convertible Preferred Stock of JanOne Inc., dated October 1, 2020 (incorporated by reference to Exhibit 3.8(a) of the Company’s Current Report on Form 8-K filed with the SEC on October 2, 2020) |
|
3.11 |
|
Bylaws of Appliance Recycling Centers of America, Inc. (incorporated by reference to Exhibit 3.4 of the Company’s Current Report on Form 8-K filed with the SEC on March 13, 2018) |
|
3.12 |
|
First Amendment to Bylaws of Appliance Recycling Centers of America, Inc. (incorporated by reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K filed with the SEC on December 31, 2018) |
|
4.1 |
|
|
|
4.2 |
|
Form of Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.2 of JanOne Inc.’s Annual Report on Form 10-K for the fiscal year ended December 28, 2019, filed with the SEC on April 6, 2020) |
|
4.3* |
|
Form of Specimen Preferred Stock Certificate |
|
4.4* |
|
Form of Certificate of Designation of Preferred Stock |
|
4.5* |
|
Form of Warrant Agreement (including Warrant Certificate) with respect to Warrants to purchase Debt Securities |
|
4.6* |
|
Form of Warrant Agreement (including Warrant Certificate) with respect to Warrants to purchase Common Stock |
|
4.7* |
|
Form of Warrant Agreement (including Warrant Certificate) with respect to Warrants to purchase Preferred Stock |
|
4.8* |
|
Form of Warrant Agreement (including Warrant Certificate) with respect to Warrants to purchase Units |
II-4
|
4.9* |
|
Form of Unit Agreement (including Unit Certificate) |
|
4.10* |
|
Form of Rights Agreement (including Form of Rights Certificate) |
|
5.1 |
|
|
|
23.1 |
|
|
|
23.2 |
|
|
|
23.3 |
|
|
|
24.1 |
|
Powers of Attorney (included on applicable signature page to this registration statement) |
|
25.1** |
|
Form T-1 Statement of Eligibility under Trust Indenture Act of 1939 of Debt Trustee (to be filed prior to any issuance of Senior Debt Securities) |
|
25.2** |
|
Form T-1 Statement of Eligibility under Trust Indenture Act of 1939 of Debt Trustee (to be filed prior to any issuance of Subordinated Debt Securities) |
|
* |
To be filed as an amendment or as an exhibit to a document filed under the Exchange Act and incorporated by reference into this registration statement. |
|
** |
To be filed in accordance with the requirements of Section 305(b)(2) of the Trust Indenture Act of 1939. |
ITEM 17. UNDERTAKINGS
The undersigned Registrant hereby undertakes:
|
|
(1) |
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:s |
|
|
(i) |
to include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
|
|
(ii) |
to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
|
|
(iii) |
to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
|
|
(2) |
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
|
|
(3) |
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
|
|
(4) |
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
|
|
(i) |
Each prospectus filed by the Registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
II-5
|
|
(ii) |
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5) or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii) or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which the prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date. |
|
|
(5) |
That, for the purpose of determining liability of the Registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
|
|
(i) |
Any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424; |
|
|
(ii) |
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant; |
|
|
(iii) |
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned Registrant; and |
|
|
(iv) |
Any other communication that is an offer in the offering made by the undersigned Registrant to the purchaser. |
|
|
(6) |
That, for purposes of determining any liability under the Securities Act of 1933, each filing of the Registrant’s annual report pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
|
|
(7) |
That, for purposes of determining any liability under the Securities Act, (i) the information omitted from the form of prospectus filed as part of the registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the registrant pursuant to Rule 424(b)(l) or (4) or 497(h) under the Securities Act shall be deemed to be a part of the registration statement as of the time it was declared effective; and (ii) each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
|
|
(8) |
To file an application for the purpose of determining the eligibility of the trustee to act under subsection (a) of Section 310 of the Trust Indenture Act in accordance with the rules and regulations prescribed by the Commission under Section 305(b)(2) of the Trust Indenture Act. |
II-6
|
|
(9) |
That, insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue. |
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Las Vegas, State of Nevada, on this 23rd day of December, 2020.
|
|
JANONE INC. |
|
|
|
|
|
|
|
|
|
By: |
/s/ Tony Isaac |
|
|
|
|
Tony Isaac |
|
|
|
|
President and Chief Executive Officer |
|
Each person whose signature appears below hereby constitutes and appoints Tony Isaac and Virland A. Johnson, and each of them, as his true and lawful attorney-in-fact and agent with full power of substitution, for him in any and all capacities, to sign any and all amendments to this registration statement (including post-effective amendments or any abbreviated registration statement and any amendments thereto filed pursuant to Rule 462(b) increasing the number of securities for which registration is sought), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
|
Signature |
Title |
Date |
|
/s/ Tony Isaac |
|
|
|
Tony Isaac |
President and Chief Executive Officer and Director |
December 23, 2020 |
|
|
(Principal Executive Officer) |
|
|
/s/ Virland A. Johnson |
|
|
|
Virland A. Johnson |
Chief Financial Officer and Executive Vice President |
December 23, 2020 |
|
|
(Principal Accounting and Financial Officer) |
|
|
|
|
|
|
/s/ Richard D. Butler, Jr. |
|
|
|
Richard D. Butler, Jr. |
Director |
December 23, 2020 |
|
|
|
|
|
/s/ John Bitar |
|
|
|
John Bitar |
Director |
December 23, 2020 |
|
|
|
|
|
/s/ Nael Hajjar |
|
|
|
Nael Hajjar |
Director |
December 23, 2020 |
II-7